ISBN 978-85-85905-19-4
Área
Química Verde
Autores
Oliveira, D.S.B.L. (UNIVERSITY OF SOUTH FLORIDA – USF) ; Oliveira, L.S.B.L. (UNIVERSITY OF SOUTH FLORIDA – USF) ; Holanda, B.B.C. (UNESP) ; Alarcon, R.T. (UNESP) ; Bannach, G. (UNESP)
Resumo
Nowadays polymers synthesized from industrial wastes have a great appreciation considering factors such as sustainability and environmental preservation. In this work, some polymers were synthesized by reacting diurethanedimethacrylate (UDMA) and glycerol, using curcumin and ethyl-p-diethylaminobenzoate as radical photoinitiators and light in the blue wavelength. Thermogravimetry- Differential Thermal Analysis, Differential Scanning Calorimetry, Vibrational Infrared Spectroscopy and Scanning Electron Microscopy were employed to study the new synthesized polymers. The use of curcumin as a photoinitiator proved to be effective. Polymers from Group II had a greater total conversion degree than did polymers from Group I. This shows that the presence of amine is not critical in polymerization.
Palavras chaves
Photopolymerization; Curcumin; Thermal Analysis
Introdução
In recent years the area of polymer studies has became increasingly important to our society due to the broad range of materials that polymers can replace in the areas of engineering, pharmaceuticals, medicine, automobiles, and textiles (Silva, 2010). In the case of photocurable polymers, that is, those that are hardened through photo induction, for instance, polymers are useful as coatings, paints, adhesives, composites, holographic devices, and optical elements (Fouassier, 2003). Among the substances that work well as photoinitiators (PI) in the visible light spectrum are dyes and pigments (Fouassier et al., 2010). Furthermore, the type II PIS of dye/amine has been widely acknowledged as an effective photopolymerization route (Fouassier et al., 2003). The photocuring processes have received special attention due to countless applications and importance in the area of materials, due to fast synthesis and easy application. As the area of polymer chemistry expands, it becomes necessary to search for novel and more efficient monomeric mixtures, and initiating systems (Mishra & Daswal, 2005;). Curcumin, an intensively yellow dye found in the rhizomes of Curcuma longa, has been previously reported as an effective PI for the copolymerization of styrene and methylmethacrylate (Mishra & Daswal, 2007) along with coinitiators such as tertiary amines or polyalcohol (glycerol). The development of technologies for synthesizing polymers from industrial waste such as residual glycerol obtained from biodiesel production is economically and technologically as strategic advantage due to the low cost of production of these compounds that otherwise might even become an environmental problem.
Material e métodos
For the preparation of the monomeric mixtures, UDMA (Urethane Dimethacrylate) (Aldrich) was added to eight individual amber flasks, each with 0.01 mol of the monomer. Then, for two of these flasks, 0.01 mol of glycerol (Merck) were added (1G); 0.03 mol (3G), 0.05 mol (5G). The photoinitiating solutions were prepared by dissolving 20 mmol Curcumin (C) (Aldrich) in acetone. For Group I samples, 20 mmol of Ethyl-p- dimethylaminobenzoate were added to the monomeric mixtures, whereas for Group II samples, no amine was incorporated. The final mixtures containing monomers and PIS were photopolymerized using the light emission equipment D-2000 (DMC Ltd., São Carlos), which uses LED to emit blue light in the range 430-490 nm. To simplify, polymers were named as PUDMAC- system number (1G, 3G and 5G). Simultaneous TG-DTA curves for each polymer were obtained using the thermal analysis system from Netzsch, model STA 449 F3. Approximately 10 mg of powdered sample were measured and placed in a 70-µL α-alumina open crucible. The parameters were set at a heating rate of 10°C/min, and a flow rate of 50 mL/min in a dry air atmosphere. The temperature range was from 30 to 800°C. DSC graphs for each polymer were obtained with a Mettler-Toledo DSC1 Stare system. Approximately 10 mg of sample were placed in a 40-µL closed aluminum crucible with perforated lid. The heating rate was of 10 °C/min and the flow rate was of 50 mL/min. The environment used was dry air atmosphere. The cycle of heating/cooling began at room temperature (25 °C). To calculate the degree of conversion for each polymer, a spectrophotometer from Bruker, model Vertex 70, was used. The equipment operated in the range 4,000-400 cm-1.
Resultado e discussão
After polymerization, the fluid monomeric mixtures of all samples became thick
and rigid, though still pliable. This hardening was the first indication that
polymerization took place. In regards to the polymers colors it was observed
that as the molar ratio of glycerol with respect to UDMA increased, the final
polymer became more whitish and turbid. In contrast, the two polymers that did
not contain glycerol were markedly more yellow than those that contained the
alcohol. TG-DTA (Figure 1) all polymers exhibited four mass loss steps. The
average thermal stability for Group I polymers was 118.5°C and that for Group
II polymers, 117.8 °C. The relatively small values from addition of glycerol,
in different ratios, had little effect on the thermal stability. The second
step was not as exothermic due the volatilization of residual monomers, which
is an endothermic process that could offset the exothermicity of the initial
degradation. DSC curves showed no thermal events could be discerned for either
the cooling or heating stages. The Results indicates that the polymers created
are thermoset. Using MIR (Figure 2) it was possible to produce conversion
degree curves for all polymers. Group II polymers had a greater total
conversion degree than their corresponding Group I polymers. Experiments have
shown that Group II’s percent increase in total conversion degree compared to
that of Group I. Another aspect to notice is that for Group I polymers the
addition of glycerol reduced the total conversion, whereas the opposite was
observed for Group II polymers. In this way, it was possible to compare the
effect that the tertiary amine has on the polymerization. In regards to the
morphology of the polymers, the addition of glycerol imparted to the polymers
a porous surface.
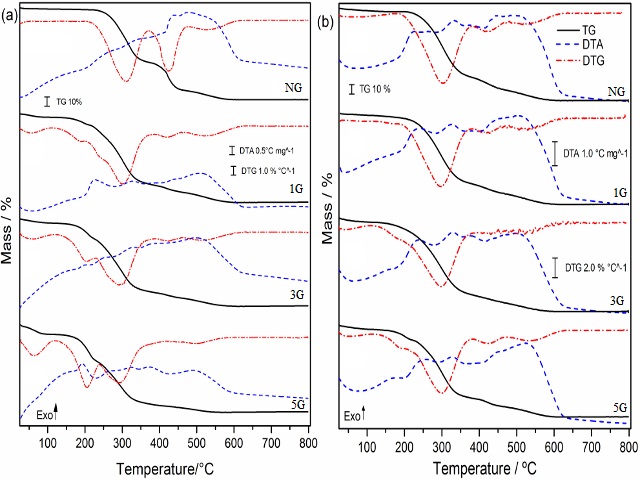
Thermal Behavior of all polymers Group I (with amine) and Group II (no amine) with different glycerol molar ratios.
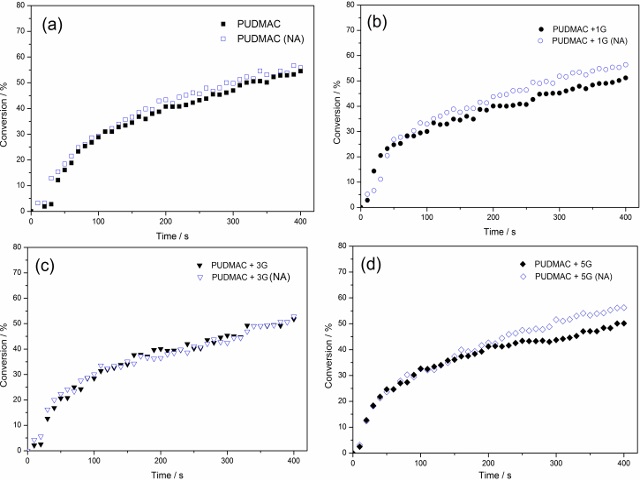
Conversion degree by MIR for all polymers. (NA) means NO AMINE
Conclusões
The use of curcumin as a photoinitiator in the polymerization of urethane dimethacrylate (UDMA) proved to be effective, with an average conversion of 53.60 % for all eight polymers studied. Likewise, the addition of glycerol onto the polymers composition did not alter their thermal stability. One interesting fact to notice is that glycerol acted not only as a co-initiator on the samples that contained it, but clearly altered the morphology of 1G, 3G, and 5G polymers. The alterations engendered by the addition of glycerol make the polymers potentially applicable as filtering materials.
Agradecimentos
FAPESP (processos: 2012/21450-1; 2013/09022-7; 2013/14884-8) CAPES Pós-graduação em Ciência e Tecnologia de materiais (POSMAT) FC-UNESP Campus de Bauru.
Referências
Fouassier, J. P., X. Allonas, and D. Burget. "Photopolymerization reactions under visible lights: principle, mechanisms and examples of applications." Progress in organic coatings 47.1 (2003): 16-36.
Fouassier, Jean-Pierre, et al. "Dyes as photoinitiators or photosensitizers of polymerization reactions." Materials 3.12 (2010): 5130-5142.
Silva, Paulo HR, Valter LC Gonçalves, and Claudio JA Mota. "Glycerol acetals as anti-freezing additives for biodiesel." Bioresource technology 101.15 (2010): 6225-6229.
Mishra, Anuradha, and Swati Daswal. "Curcumin, A Novel natural photoinitiator for the copolymerization of styrene and methylmethacrylate." Journal of Macromolecular Science, Part A 42.12 (2005): 1667-1678.
Mishra, Anuradha, and Swati Daswal. "Curcumin, a natural colorant as initiator for photopolymerization of styrene: kinetics and mechanism." Colloid and Polymer Science 285.10 (2007): 1109-1117.
Quispe, César AG, Christian JR Coronado, and João A. Carvalho Jr. "Glycerol: Production, consumption, prices, characterization and new trends in combustion." Renewable and Sustainable Energy Reviews 27 (2013): 475-493.
Rodrigues, Rodrigo Augusto, and José Honório Accarini. "Brazil’s Biodiesel Program." Ministry of External Relations, Government of Brazil. http://www. dc. mre. gov. br/imagens-e-textos/Biocombustiveis-09ingprogramabrasileirobiodiesel. pdf (2009).
Da Silva, Gervásio Paulo, Matthias Mack, and Jonas Contiero. "Glycerol: a promising and abundant carbon source for industrial microbiology." Biotechnology advances 27.1 (2009): 30-39.











