ISBN 978-85-85905-19-4
Área
Química Verde
Autores
Garcia-martinez, E. (UNIVERSIDAD DE VALENCIA) ; Passos, F. (UNIVERSIDADE FEDERAL DA BAHIA) ; Yuste, A. (UNIVERSIDAD DE VALENCIA) ; Martínez-navarrete, N. (UNIVERSIDAD DE VALENCIA) ; Iglesias, M. (UNIVERSIDADE FEDERAL DA BAHIA)
Resumo
Acerola fruit (Malpighia emarginata) is well known as an excellent food source of vitamin C, and it also contains phytochemicals such as carotenoids and polyphenols which significantly contribute to its total antioxidant capacity. The extractions and analyses of the bioactive substances present in fruits involve techniques that use organic solvents that are highly toxic to human health and environment. To improve the safety and environmental friendliness of conventional separation techniques, Protic Ionic Liquids (PIL’s) were used as ideal substitutes because of their stability, nonvolatility and adjustable miscibility and polarity. The protic ionic liquid 2HDEAPr gathered the best results as additive solvent in the extraction of bioactive compounds.
Palavras chaves
Protics Ionics Liquids; Antioxidants; Acerola
Introdução
Fruits, in particular the tropical ones, are aliments of great interest for human nutrition due to their high nutritious and therapeutic properties. (Schieber, 2001) Studies provide evidences that the ingestion of foods that are rich in natural compounds promotes the stabilization of free radicals (antioxidant capacity) in the human body and reduces infirmities which are of great concern, such as cancer and cardiovascular diseases. (Halliwell, 2007, Food Ingredients Bazil, 2015) The acerola, a tropical fruit found in Brazil roughly 60 years ago, is cultivated especially in the country’s northeastern region, markedly in the states of Pernambuco, Paraíba, Bahia and Ceará. It poses itself as a fruit richly laden with vitamins, e.g. ascorbic acid (Vitamin C), carotene (Vitamin A), thiamine (Vitamin B1), riboflavin (Vitamin B2), niacin and mineral salts (iron, calcium, phosphorus) and sugars, besides also being rich in bioactive substances (carotenoids and phenolic compounds) that grant it great antioxidant power. The high concentrations of ascorbic acid confer the acerola the ability to prevent fatigue and appetite loss, diminishing one’s odds of contracting infectious diseases and suffering from muscular and joint pains. However, despite its nutritious relevance, the acerola is yet to be amply studied (Yamashita, 2003). The productivity and quality of the acerola fruits have been perfected throughout the last few years, having the processing industries of Brazil absorbed most of the national production in the forms of pulps, frozen fruits and pasteurized juices. Both the internal and external markets have shown an ever-increasing demand, not only with respect to the quantities of the products but also to their diversification. Hitherto there haven’t been developed nutraceutical initiatives that are able to bring forth all of the potential that the acerola fruit bears, a fact that makes the investigation of new extraction techniques and possible applications of the obtained bioactives relevant. (Assis et al., 2001) In this context blossoms the so-called Green Chemistry, often defined as the application of the science and production of chemicals in a sustainable, safe and non-pollutant manner, consuming the least possible quantities of raw materials and energy while emitting no residues (Erias Rey and Alvarez-Campana Gallo, 2007). The usage of the protic ionic liquids (PILs) in extraction processes poses itself as an excellent alternative, since those new compounds confer a less potentially toxic load while promoting a highly efficient extraction, bearing greater selectivity and being biodegradable substances. The use of protic ionic liquids also promotes lower energy consumption: they are easily separable, can be reused as of the end of the process, have low costs and are of particularly easy synthesis. In this fashion, the processes become less toxic, safer and thus more sustainable with respect to the management of edibles (Reis et al., 2012). These substances are characterized as non-volatile organic salts, and this trait prevents their dispersion through evaporation. They have recently been the object of studies that demonstrate their myriad of social and industrial application (Adams et al., 1998, Cull et al., 2000, Baston et al., 2002, Zhao et al., 2002, Lei et al., 2003, Welton, 2004, Zhou, 2005, Liu et al., 2005, Zhao et al., 2005, Li et al., 2007, Sun et al., 2007, Borra et al., 2007, Hagiwara and Lee, 2007, Nakamoto and Watanabe, 2007, Cieniecka-Roslonkiewicz et al., 2007, Hough et al., 2007). An important aspect that is yet to be studied in sufficient depth is the application of the protic ionic liquids as selective extractors of biocompounds. In the field of food technology, some studies support the application of ionic liquids in the extraction of antioxidants stage as well as its use as the stationary phase in the chromatographic analysis (Torrecilla, 2012). This work aims to study and assess the effects of the addition of protic ionic liquids to the solvents employed in the extraction (water and methanol) of the acerola’s antioxidant compounds through the DPPH method. (Brand-Williams et al., 1995)
Material e métodos
Synthesis and Preparation of Ionic Liquids The protic ionic liquids were synthesized through simple Bronsted-Lowry neutralization reactions, in which the acid acts as proton donor and the base as its acceptor. Two bases were used in this work (monoethanolamine and diethanolamine) which were combined with a collection of organic acids (acetic acid, propionic acid and salicylic acid), resulting in six ionic liquids of distinct natures and properties. The chemical reactions are of exothermic character, thus demanding a strict temperature control to avoid thermal degradation. The purification was conducted under constant stirring and slight heating over 48 hours, assuring the thorough elimination of the residual reactants and of the absorbed water. Sample Preparation The acerola pulp samples were provided from local provider. 2 g of the pulp that was diluted in different solvents were used in order to assess its antioxidant capacity. The utilized solvents were: pure methanol, pure water, a mixture of methanol with 10% of each protic ionic liquids and a mixture of water with 10% of protic ionic liquids. The mixture of pulp and solvents underwent agitation for 20 minutes, being centrifuged short after at the conditions of 800 rpm throughout 10 minutes and at a temperature of 20° C. After the centrifugation, the obtained samples were filtered at 0.45 µm in order to finally be submitted to the analyses of their antioxidant capacities through the DPPH method.
Resultado e discussão
Determination of the Antioxidant activity through the use of the DPPH
method.
The method is based on the methodology described by Brand-Williams et al
(1995),
in which the DPPH radical undergoes reduction and the color change is
proportional to the antioxidant capacity of the studied compound. 3 mL of
the
fruit sample were added to 1 mL of methanolic solution of DPPH at (6x10–5M).
For
the sake of solution control ethanol was used as the white. As the standard
reference solution the Trolox solution was employed (6-Hydroxy-2,5,7,8-
tetramethylchromane-2-carboxylic acid) at 97%. The mixture was homogenized
and
past 30 minutes of rest it underwent a reading effectuated through the use
of a
UV spectrophotometer at the wavelength of 517 nm. Further readings were
conducted at intervals of five minutes until the results reached a stable
value.
From the absorbance data gathered the calculations of DPPH concentration
were
effectuated stemming from a calibration curve obtained through linear
regression. The results were expressed in an activity equivalent to the
Trolox
(µM/g of fruit).
In rows, different letters denote significant differences (p <0.05)
Results demonstrate that in all cases, the addition of PIL’s significant
increased (p <0,05) the values of antioxidant activity of acerola. Methanol
fractions provided significant (p <0,05) better antioxidant activity results
than water. In all cases, 2HDEASa extractions exhibited the highest (p
<0,05)
antioxidant activity, thus reducing the amount of methanol employed in the
extraction. It seems feasible to use PIL’s to reduce the use of toxic and
harmful organic reagents for the environment and to improve the extraction
step
phytochemicals.
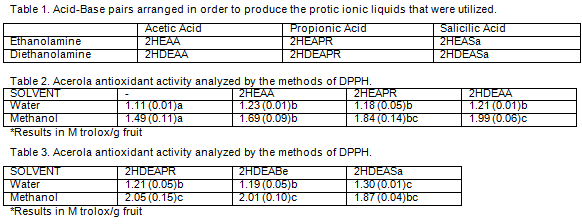
Acid-Base pairs arranged in order to produce the protic ionic liquids that were utilized/. Acerola antioxidant activity analyzed by the methods of DPP
Conclusões
Attending to previously results, as expected, protic ionic liquids gathered an amazing capability as selective solvent for antioxidant compounds from acerola. In all tested samples, the protic ionic liquid 2HDEASa showed the best results, reducing the use of traditional organic solvents, improving a promising alternative procedure for nature phytochemicals extraction processes.
Agradecimentos
Special thanks are owed to the FAPESB, due to the scholarship it provided, and to the PROPCI, for its invaluable financial support.
Referências
Adams CJ, Earle MJ, Roberts G, Seddon KR “Friedel-Crafts reactions in room temperature ionic liquids”, CHEMICAL COMMUNICATIONS (19): 2097-2098, 1998
Assis, S.A.; Lima, D.; Faria Oliveira, O. (2001). Activity of pectinmethylesterase, pectin contente and vitamin C in acerola fruit at various stages of fruit development. Food Chemistry. 2: 133-137.
Baston GMN, Bradley AE, Gorman T, Hamblett I, Hardacre C, Hatter JE, Healy MJF, Hodgson B, Lewin R, Lovell KV, Newton GWA, Nieuwenhuyzen M, Pitner WR, Rooney AW, Sanders D, Seddon KR, Simms HE, Thied RC, “Ionic liquids for the nuclear industry: A radiochemical, structural, and electrochemical investigation”, IONIC LIQUIDS ACS SYMP. SERIES 818: 162-177 2002
Borra EF, Seddiki O, Angel R, Eisenstein D, Hickson P, Seddon KR, Worden SP,“Deposition of metal films on an ionic liquid as a basis for a lunar telescope”,NATURE 447, 979-981, 2007
Brand-Williams, W.; Cuvulier, M.E.; Berset, C. (1995). Use of free radical method to evaluate antioxidant activity. Lebesnsm.U.-Technol. 28: 25-30.
Cieniecka-Roslonkiewicz A, Sas A, Przybysz E, Morytz B, Syguda A, Pernak J, “Ionic liquids for the production of insecticidal and microbicidal extracts of the fungus Cantharellus cibarius”, CHEMISTRY & BIODIVERSITY 4 (9): 2218-2224, 2007
Cull SG, Holbrey JD, Vargas-Mora V, Seddon KR, Lye GJ, “Room-temperature ionic liquids as replacements for organic solvents in multiphase bioprocess operations”, BIOTECHNOLOGY AND BIOENGINEERING 69 (2): 227-233, 2000
Erias Rey A.; Alvarez-Campana Gallo J.M. 2007. Evaluación ambiental y desarrollo sostenible. Ed. Pirámide
Food Ingredients Bazil. Dossiê Antioxidantes: Antioxidantes. São Paulo: Editora Insumos, v. 33, 06/2009. Mensal. Disponível em: <http://www.revista-fi.com/materias/83.pdf>. Acesso em: 24/07/2015
Hagiwara R, Lee JS, “Ionic liquids for electrochemical devices”, ELECTROCHEMISTRY 75 (1): 23-34, 2007
Halliwell, B. (2007). Dietary polyphenols: good, bad, or indiferente for your health?” Cardiovascular Research, 73 (2): 341–347.
Hough WL, Smiglak M, Rodriguez H, Swatloski RP, Spear SK, Daly DT, Pernak J, Grisel JE, Carliss RD, Soutullo MD, Davis JH, Rogers RD “The third evolution of ionic liquids: active pharmaceutical ingredients”, NEW JOURNAL OF CHEMISTRY 31 (8): 1429-1436, 2007
Lei ZG, Li CY, Chen BH, “Extractive distillation: A review”, SEPARATION AND PURIFICATION REVIEWS 32 (2): 121-213, 2003
Li ZJ, Chang J, Shan HX, Pan JM, “Advance of room temperature ionic liquid as solvent for extraction and separation”, REVIEWS IN ANALYTICAL CHEM. 26 (2): 109-153, 2007
Liu JF, Jonsson JA, Jiang GB, “Application of ionic liquids in analytical chemistry”, TRAC-TRENDS IN ANALYTICAL CHEMISTRY 24 (1): 20-27, 2005
Nakamoto H, Watanabe M, “Bronsted acid-base ionic liquids for fuel cell electrolytes”, CHEMICAL COMMUNICATIONS (24): 2539-2541, 2007
Reis, I.A.O.; Santos, S.B.; Santos, L.A.; Oliveira, N.; Freire, M.G.; Pereira. J.F.B.; Ventura, S.P.M.; Coutinho, J.A.P.; Soares, C.M.F.; Lima, A.S. (2012) Increased significance of food wastes: Selective recovery of added-value compounds. Food Chem. 135: 2453.
Schieber, A.; Stintzing, F.C.; Carle, R. (2001). By-products of plant food processing as a source of functional compounds-recent development. Trends Food Sci. Technol. 12: 401-413.
Sun XQ, Xu AM, Chen J, Li DQ, “Application of room temperature ionic liquid-based extraction for metal ions”, CHINESE J. OF ANALYTICAL CHEMISTRY 35 (4): 597-604, 2007
Torrecilla, J. S. 2012. The Role of Ionic Liquids in the Chemical Industry.Nova Publishers. NY.
Welton T, “Ionic liquids in catalysis”, COORDINATION CHEMISTRY REVIEWS 248 (21-24): 2459-2477, 2004
Yamashita, F.; Benassi, M. T.; Tonzar, A.C.; Moriya, S.; Fernandes, J.G. 2003. Produtos de acerola: estudo da estabilidade de vitamina C. Ciencia e Tecnologia de Alimentos, 23(1), 92–94
Zhao DB, Wu M, Kou Y, Min E, “Ionic liquids: applications in catalysis”, CATALYSIS TODAY 74 (1-2): 157-189, 2002
Zhao H, Xia SQ, Ma PS, “Use of ionic liquids as 'green' solvents for extractions”, JOURNAL OF CHEMICAL TECHNOLOGY AND BIOTECHNOLOGY 80 (10): 1089-1096, 2005
Zhou Y, “Recent advances in ionic liquids for synthesis of inorganic nanomaterials”, CURRENT NANOSCIENCE 1 (1): 35-42, 2005











