Synthesis and cytotoxicity of 2,6,9-trisubstituted purines derivatives
ISBN 978-85-85905-21-7
Área
Química Orgânica
Autores
Espinosa-bustos, C. (PONTIFICIA UNIVERSIDAD CATOLICA DE CHILE) ; Salas, C. (PONTIFICIA UNIVERSIDAD CATOLICA DE CHILE) ; Faundez, M. (PONTIFICIA UNIVERSIDAD CATOLICA DE CHILE) ; Gallardo, C. (PONTIFICIA UNIVERSIDAD CATOLICA DE CHILE)
Resumo
A series of 2,6,9-trisubstituted purine derivatives have been synthesized and investigated for their potential role as antitumor agents. All compounds were evaluated in vitro to determine their potential effect on cell toxicity by the MTT method on three cancer cells lines and Vero cells. Nine out of ten compounds were found to be promising agents compared to a known and effective anticancer drug, etoposide on HeLa (Human cervix adenocarcinoma) and HL-60 (Human promyelocytic leukaemia) cells.
Palavras chaves
purines; cytotoxicity; MTT assay
Introdução
Purine scaffold is often found in several molecules with biological properties, receiving the title of privileged scaffold. Therefore, the purine core is an interesting structural fragment that has been incorporated into new drugs. Some examples of major purine-based drugs that are currently being used are: anticancer agents (6-mercaptopurine, thioguanine), antiviral agents to overcome infections such as herpes or AIDS (acyclovir, ganciclovir, carbovir, abavavir), among others. In recent years, several di- and tri-substituted purine derivatives have been synthesized and tested on cancer cells. Furthermore, purine’s 2,6,9-substitution pattern is found in several compounds with anti-cancer properties such as myoseverin and roscovitine. Considering the importance of having new anticancer therapies, in this work we show the synthesis and cytotoxic evaluation of tri- substituted purine derivatives.
Material e métodos
2,6-Dichloropurine, benzyl amines and the alkyl halides, were purchased from Sigma-Aldrich (St. Louis, MO, USA). Cell culture medium, fetal bovine serum and penicillin-streptomycin were purchased from Biological Industries (Kibbutz Beit Haemek, Israel). Etoposide, 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyl tetrazolium bromide (MTT) and all other chemicals were obtained from Sigma- Aldrich. Nuclear magnetic resonance spectra were recorded on a Bruker AM-400 apparatus using CDCl3 solutions containing TMS as internal standard. Thin layer chromatography (TLC) was performed using Merck GF-254 type 60 silica gel. Column chromatography was carried out using Merck silica gel 60 (70–230 mesh).
Resultado e discussão
Chemistry
To carry out the synthesis of compounds 4a-l, 6-chloro-2-fluoropurine 1 was
used as starting material, according to Scheme 1. First, through a reaction
of N-alkylation using benzyl chloride, the derivative substituted at
position 9 of the purine 1, with 48 % yield it was achieved. Additionally,
was obtained the regioisomer alkylated at the 7 position with a 13 % (not
shown). Later, position 6 of purine 1 core was substituted with
trifluoromethoxyphenyl group by Suzuki-Miyaura C-C coupling using the
respective boronic acid and Pd(PPh3)2Cl2 as palladium source under dioxane
reflux, furnishing the compound 3 with a 67 % yield. Finally, derivatives
4a-l were obtained in good yields (43 – 94 %) using 12 different
benzylamines, n-butanol as solvent and DIPEA. All compounds (finals and
intermediates) were characterized by NMR spectroscopy of 1H, 13C and 19F, IR
spectra and high-resolution mass spectres. The final compounds 4a-l shown as
characteristic signals the proton of NH group in the zone of 5.50 – 5.70 ppm
as a broad signal, and the hydrogens of the benzylamine methylene group at
4.60 – 4.80 ppm as a doublet signal.
Biology
The potential in vitro antitumor activity of compounds 4a–l was initially
tested for their cytotoxic effects on HL-60, HCT116 and HeLa cancer cell
lines and Vero cells. A conventional colorimetric assay was set up to
estimate the IC50 values, which represent the concentration of a drug that
is required for 50 % inhibition in vitro after 72 h of continuous exposure
to compounds. Four serial dilutions (from 12.5 to 100 μM) for each sample
were evaluated in triplicate and etoposide was used as a positive control
and reference drug.
In general, all compounds showed high cytotoxicity in cancerous cells and
low toxicity in Vero cells.
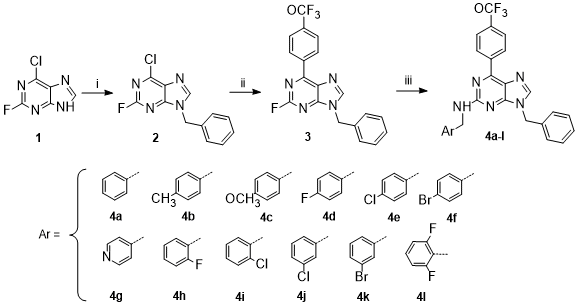
Scheme 1. Reagents and conditions: i) benzyl bromide, K2CO3, DMF, 6 h., r.t. ii) trifluoromethoxy phenyl boronic acid, Pd(PPh3)2Cl2, K2CO3 2M, Dioxane, 2h, reflux. Iii) benzylamines, DIPEA, n-BuOH, 12 h, reflux.
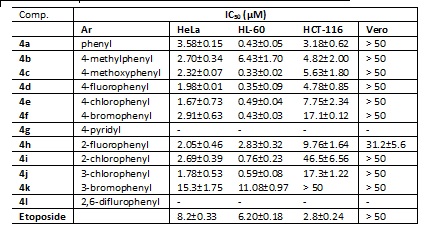
Table 1. In vitro cytotoxicity of compounds 4a–4l on cancer cell lines and Vero cells.
Conclusões
New 2,6,9-trisubstituted purines was synthesized and evaluated their cytotoxicity on three cancer cell lines and Vero cells. The vast majority of these compounds were more potent than the reference drug etoposide and exhibited selectivity against three cancer cell lines in comparison with VERO cells. These results showed that these compounds are promising leads for the development of new antitumor drugs.
Agradecimentos
FONDECYT(Postdoctoral fellowship 3150198)
Referências
Calderon-Arancibia, J. et al. Molecules 2015, 20, 6808-6826.
































