Design and Synthesis of New 9-Alkyl-6-Aryl-2-Piperidinyl Benzamide Purine Derivatives
ISBN 978-85-85905-21-7
Área
Química Orgânica
Autores
Villegas, A. (PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE) ; Salas, C.O. (PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE)
Resumo
2,6,9-trisubstituted purine derivatives exhibited cytotoxicity and selectivity in several cancer cell lines. Therefore we designed and synthesized 34 new 2,6,9-trisubstituted purines, through a simple strategy of convergent synthesis in five step, with good yields. These compounds are being studied their cytotoxicity in cancer cell lines with the goal to develop new antitumor agents.
Palavras chaves
purine derivatives; organic synthesis; piperidinidyl benzamides
Introdução
Cancer is one of the most important problems in public health, and is the main disease responsible for deaths worldwide. Currently cancer chemotherapy represents a constant, global and interdisciplinary research effort because it is an urgent need to promote and extend the quality of life for the world population. New antitumor therapies point towards new compounds that demonstrate selective anticancer actions, and thus must exhibit a cytotoxic effect on malignant cells, without damaging normal cells. Most of the known synthetic anticancer drugs are heterocyclic compounds, and several of them correspond to nitrogen heterocycles. On the other hand, the purine nucleus is present in several biological molecules that play a key role in signaling pathways, metabolism and other cellular processes in all organisms, due that, in medicinal chemistry is called a "privileged scaffold". Additionally, some examples involving the purine fragment are anticancer agents (6-mercaptopurine, thioguanine), antiviral agents (acyclovir), among others. Some authors have reported that 2,6,9- trisubstituted purine derivatives exhibited promising cytotoxicity and selectivity in some cancer cell lines,1 as well as, some tetrazine derivatives that containing the piperidine benzamide moiety3 (Figure 1). Therefore, based on compounds I-III,1,3 we designed and synthesized a new series of 2,6,9-trisubstituted purine derivatives with potential antitumor activity.
Material e métodos
Reagents and solvents used for the synthesis were of analytical grade, and each compound (final and intermediates) was characterized by means of NMR spectroscopy of 1H, 13C, 19F in a Bruker model AM-400, FT-IR with a Bruker model Vector 22. The synthetic methodology correspond to classic organic reactions which are developed in our group and reported in previous work.
Resultado e discussão
First, compounds 3a and 3b and their respective regioisomers (not shown in
the Scheme 1), were synthesized from an N-alkylation reaction to a
commercial purine 1 with two different alkyl halides. The Suzuki-Miyaura
coupling reaction was then performed just with regioisomers 3a,b with 4-
trifluoromethoxyphenyl boronic acid to yield 4a,b respectively in good
yields (65 and 80 %). In parallel, the synthesis of compound 5a-q was
obtained by reaction of 17 different aromatic acid chlorides and 4-amino-1-
boc-piperidine 6, and then deprotection of Boc group is performed in acid
medium. Finally, a nucleophilic aromatic substitution in position 2 of the
purine 4a,b and the respective piperidine-benzamides 8a-q, was carried out
to obtain final products 9a-q and 10a-q were obtained with good yields.
The biological evaluation of all final compounds is ongoing performed by the
MTT assay at several concentrations in time of 72 hours in some cancer cell
lines, as well as, in VERO healthy cells.
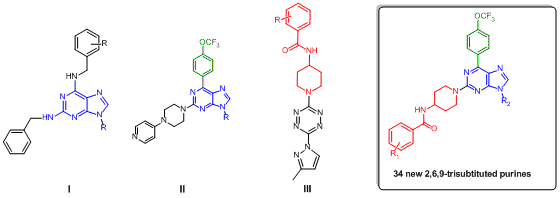
Chemical structures of purine and tetrazine derivatives with anticancer activity and the target compounds.
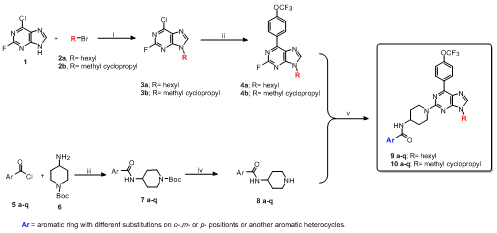
i) K2CO3, DMF,6h, rt; ii)boronic acid, Pd(PPh3)Cl2, K2CO3, dioxane, 2h, reflux; iii) Et3N, THF, 3h, rt; iv)TFA/CH2Cl2; v)DIPEA, n- butanol, 12h, reflux
Conclusões
Through of well-known organic synthetic procedures, were obtained 34 new 2,6,9-trisubstituted purine derivatives, using a simple strategy of convergent synthesis in five step. Biological study are being developed to determinate the eventual antitumor activity on several cancer cell lines.
Agradecimentos
We gratefully acknowledge the financial support of FONDECYT (project 1161816) and CONICYT-PCHA/National Ph.D./2015-21150586.
Referências
1.Cañete-Molina, Á.; Espinosa-Bustos, C.; González-Castro, M.; Faúndez, M.; Mella, J.; Tapia, R.A.; Cabrera, A.R.; Brito, I.; Aguirre, A.; Salas, C.O. Arabian Journal of Chemistry 2017, http://dx.doi.org/10.1016/j.arabjc.2017.04.002
2.Bach, P. B.; Mirkin, J. N.; Oliver, T. K.; Azzoli, C. G.; Berry, D. A.; Brawley, O. W.; Byers, T.; Colditz, G. A.; Gould, M. K.; Jett, J. R.; Sabichi, A. L.; Smith-Bindman, R.; Wood, D. E.; Qaseem, A.; Detterbeck, F. C. Jama 2012, 307 (22), 241.
3.Graham, P. An Introduction to Medicinal chemistry, 5ta editio.; Oxford, Ed.; Oxford, 2013.
4.Calderón-Arancibia, J.; Espinosa-Bustos, C.; Cañete-Molina, Á.; Tapia, R. A.; Faúndez, M.; Torres, M. J.; Aguirre, A.; Paulino, M.; Salas, C. O. Molecules 2015, 20 (4), 6808–6826.
































