Synthesis and cytotoxic studies of new 2-anilines-6-aryl-alkyl- purines derivatives
ISBN 978-85-85905-21-7
Área
Química Orgânica
Autores
Daniela, I. (PUC/CHILE) ; Cristian, S. (PUC/CHILE) ; Marcelo, M. (PUC/CHILE) ; Ana Maria, E. (PUC/CHILE) ; Christian, E. (PUC/CHILE)
Resumo
The purine core is an interesting heterocycle in medicinal chemistry because it is considered a privilege scaffold. Its versatility allows the synthesis of several compounds with different biological properties. One of the diseases that has been a focus for testing these compounds is cancer. In our research group, through a fragment-based design, we have proposed the synthesis of 2,6,9-trisubstituted purines derivatives with cytotoxic effects on four cancer cell lines. In this work, we show the synthesis of six derivatives and their biological evaluation by MTT assay in order to determinate the potential anticancer effect.
Palavras chaves
PURINES; CANCER; MEDICINAL CHEMISTRY
Introdução
Medical chemistry aims at the synthesis and development of new bioactive compounds, which may have a potential therapeutic use. One of the challenges in this area is the creation of more potent and specific molecules that can improve existing pharmacological therapies. The latter is one of the main needs in cancer treatment, since the current drugs are characterized by their large number of side effects and little specificity (AVENDAÑO and MENENDEZ,2008). In the search for structures for the design of new drugs, the purine nucleus has been extensively investigated because it is present in a large number of molecules with biological activity, such as antiviral agents, anticancer agents, among others (CALDERON-ARANCIBIA et al, 2015). In addition, 2,6,9-trisubstituted purine derivatives have been shown to have antiproliferative effect, examples of which are Myosoeverine and Roscovitine (CHANG et al, 2001). For this reason, our research team, using fragment- based design, proposes that 2,6,9-trisubstituted purines may have a potential anticancer effect. In the present work, the synthesis and cytotoxic evaluation of six 2,6,9-trisubstituted purine derivatives was performed.
Material e métodos
Reagents and solvents used for the synthesis were of analytical grade, and each compound was characterized by means of NMR spectroscopy of 1 H, 13 C, 19 F in a Bruker model AM-400. The cancer cell lines AsPC-1, MCF-7, HL60, HepG2 and VERO healthy cells were maintained in RPMI 1640 medium at pH 7.4 supplemented with 10 % inactivated fetal bovine serum, 100 ug/mL penicillin, 100 ug/mL of streptomycin, non-essential amino acids and 2 g of sodium bicarbonate. Cells were maintained in a humidified incubator at 37 ° C in the presence of 5 % CO 2 . Biological assays were carried out with cultures with 80% confluency. The evaluation of the cytotoxic effect of the synthesized ligands was performed by the tetrazolium salts colorimetric assay of MTT (3- [4,5-dimethylthiazol- 2-yl] -2,5- diphenyltetrazol), where the concentration of compounds ranges from 0,05 uM to 50 uM, using etoposide as control.
Resultado e discussão
Chemistry
According to Scheme 1, the synthesis of derivatives 4a-f was performed in
three steps. First, from the commercial substrate 2,6-dichloropourine 1 by
an N-alkylation reaction using methylcyclopropyl bromide, product 2 was
obtained in 40% yield and to its regioisomeric product substituted in N-7
with a 12% yield. Then, the trifluoromethoxyphenyl boronic moiety was added
to the C-6 of derivative 2 by a Suzuki-Miyaura carbon-carbon coupling
reaction, yielding derivative 3 in 70% yield. Finally, compounds 4a-f are
obtained through the Buchwald-Hartwig amination reaction by replacing the
chlorine atom in position 2 with the respective aniline derivatives.
All final products were obtained with excellent yields, 90-96%. The final
compounds and intermediates were characterized by 1H, 13C and 19F NMR
spectroscopy.
Biology
In order to determine the cytotoxicity of these compounds, a fixed
concentration (25 μM) of all 6 molecules was screened in leukemia cell lines
(HL-60), breast cancer (MCF7), pancreatic cancer (ASPC1) and liver cancer
(HEPG2), using the VERO cell line as a reference. From this screening the
compounds 4d and 4e were the ones that achieved better results, so they were
selected to measure their IC50 values in the cell lines mentioned above
(Table 1). The screening and measurement of IC50 was performed through
tetrazolium salt reduction (MTT) colorimetric measurements.
Table 1 shows the results of the IC50 values for compounds 4d and 4e.
According to the National Cancer Institute (INC) the IC50 values to consider
a compound as active are under 10-15 μM. Considering this, the compound 4d
satisfy this characteristic in 3 of the 4 cancer lines tested, whereas 4e
only does so in the leukemia cell line. In addition, both prove to be
deficient in liver cancer
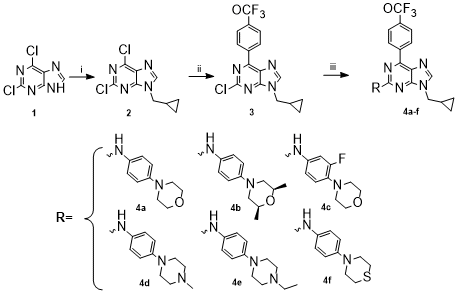
Reagents and conditions i) methylcyclopropyl bromide, K2CO3, DMF, 6 h., r.t. ii) trifluoromethoxyphenyl boronic acid, Pd(PPh3)2Cl2, K2CO3 2M, dioxane, 2h, reflux. Iii) anilines, Xantphos, Pd(OAc)2Cl2, 24 h, reflux
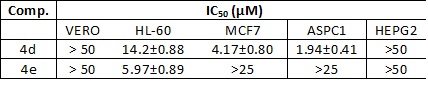
IC 50 values for compound 4d and 4e in Vero cell lines, leukemia (HL-60), breast cancer (MCF7), pancreatic cancer (ASPC1), and liver cancer (HEPG2).
Conclusões
In this work, six novel 2,6,9-trisubstituted purine derivatives were synthesized and their cytotoxicity were evaluated. According to the results obtained, only the compound 4d showed good results according to the reference values of the national cancer institute. New derivatives are being synthesized at this time, considering compound 4d as the leader.
Agradecimentos
FONDECYT project N° 1161816.
Referências
AVENDAÑO, C.; MENENDEZ, J.C. Medicinal chemistry of anticancer drugs; 2.ed. Madrid, Spain. Ed. Elsevier, 2008. P6-9.
CALDERÓN-ARANCIBIA, J.; ESPINOSA-BUSTOS, C.; CAÑETE-MOLINA, A.; TAPIA, R.A.; FAÚNDEZ, M.; TORRES, M.J.; AGUIRRE, A.; PAULINO, M. and SALAS, C.O. Synthesis and Pharmacophore Modelling of 2,6,9-Trisubstituted Purine Derivatives and Their Potential Role as Apoptosis-Inducing Agents in Cancer Cell Lines. Molecules, v. 20, p. 6808-6826, 2015.
CHANG, Y.T.; WIGNALL, S.M.; ROSANIA, G.R.; GRAY, N.S.; HANSON, S.R.; SU, A.I.; MERLIE, J., Jr.; MOON, H.S.; SANGANKAR, S.B.; PEREZ, O.; et al. Synthesis and biological evaluation of myoseverin derivatives: Microtubule assembly inhibitors. J. Med. Chem. v. 44, p. 4497–4500, 2001.
































