ÁREA: Materiais
TÍTULO: HETEROMORPHIC HEMATITE PIGMENTS FROM STEEL SCRAP ENCAPSULATED IN AMORPHOUS SILICA OBTAINED FROM RICE HUSK
AUTORES: V. P. DELLA (IFES) ; J. A. JUNKES (UFSC) ; A. P. N. OLIVEIRA (UFSC) ; L. B. CALIMAN (IFES) ; D. HOTZA (UFSC)
RESUMO: The aim of this study was to investigate the possibility of recycling steel scrap and rice husk as alternative raw materials for the synthesis of an encapsulated pigment based on iron oxide and silica for application in the ceramic industry. For the pigment synthesis, the following compositions were defined: 5, 10 and 15 wt% of cromophorous powder (95, 90 and 85 wt% of amorphous silica), which were homogenized and calcinated from 1050 to 1150ºC for 2 h. The pigments showed stability after firing, turning into cream and light pink colors with different saturations according to the added percentage and inherent amount of cromophorous material for each composition.
PALAVRAS CHAVES: heteromorphic pigments, rice husk, steel scrap
INTRODUÇÃO: Pigments are used in the production of ceramic floor and wall ceramic tiles, either in glaze preparation or in porcelainized stoneware bodies, giving decorative and esthetical properties to the tiles, giving them an enjoyable look and frequently masking their defects [1].
Researches about heteromorphic pigments, also known as encapsulated or occlusion pigments, have been one of the most promising areas on the ceramic pigment field [2, 3]. This technique consists in encapsuling the chromophorous ion inside a ceramic matrix, highly stable both chemically and thermally, which will protect it from the attack of the components from the medium where it may be dispersed. The encapsulation takes place by sintering the matrix surrounding the chromophorous material [4, 5].
Enviromental issues have influenced researches in order to improve the utilization of raw materials, minimizing the production of wastes and recycling them. One of the current trends is the search for alternative and less expensive raw materials. An alternative source for the production of iron oxide pigments is the use of steel scrap from plants of steel production and silica derived from the beneficiation of rice husk.
In this study the silica will act as matrix and the sources of hematite, as chromophorous material. The encapsulating model needs an adequate synchronism between the crystallization and sintering of silica and the growth-nucleation of hematite [6, 7].
The goal of this work is to detail the obtainment of the ceramic pigment of iron oxide III (hematite) encapsulated in silica matrix using for this alternative raw materials such as rice husk and steel scrap. The colorimetrical development will be study as well as the encapsulating percentage of the developed compositions.
MATERIAL E MÉTODOS: The experimental procedures used on the rice husk and steel scrap conversion into active silica and iron oxide III, respectively, as well as a characterization of these materials can be obtained in [8, 9].
The rice husk leaching process resulted in silica with an average particle size of 4.1 microns, approximately 98 wt% amorphous silica and specific surface area of 406.3 m2/g. The sample of steel scrap treated at 800ºC for 2 h in oxidizing atmosphere, gives origin to an iron oxide with red tonality, around 97 wt% hematite.
The pigment samples were formulated varying the content of silica from 85, 90 to 95 wt% and hematite from 5,10 to 15 wt%, identified by the letter “P” followed by the hematite percentage used on the formulation.
After weighing the corresponding fraction for each oxide, the raw materials were wet-milled in acetone in a high rotation (900 rpm) Al2O3-ball mill (Gabbrielli SRL, Mill 2) for 4 h. The prepared suspensions were dried at 110ºC (Ceramic Instruments SRL) and deagglomerated for 3 min.
The dry and deagglomerated samples were calcined at 1050 and 1150ºC for 2 h in air with a heating rate of 10ºC/min. The cooling was carried out inside the oven up to environment temperature. The calcined pigments at 1050ºC were named “A” and those ones calcined at 1150ºC were named “B”. The pigments were then grinded until a granulometry under 10 microns was reached.
In this procedure, 1 ml of HCl (Fmaia, 37 vol%) concentrated was added to 20 mg of each calcined pigment, and the volume of the solution was completed to 100 ml with deionized water. The mixture was then heated during 5 min at 50ºC, filtered, and the leached solution containing the ion iron was analyzed by atomic absorption (Zeiss, AAS4).
RESULTADOS E DISCUSSÃO: All the synthesized pigments presented the same crystalline phases: hematite and cristobalite, this was also observed by [10]. It is considered that the pigment corresponds to the hematite particle encapsulated in a cristobalite matrix.
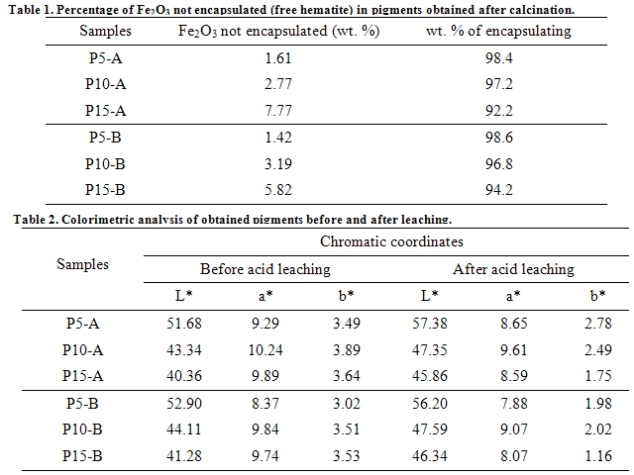
Leaching tests with HCl solutions were effective in evaluating the efficiency of the hematite encapsulating process in the sintered silica agglomerated due the fact that hematite is soluble in HCl and silica is not. The leached hematite percentage is proportional to the amount of iron percentage in each composition, resulting in an encapsulating efficiency between 92.2 and 98.6%.
Hematite normally develops a red tonality, but due to the small percentage used, the resulting color was a light pink. The calcined pigments at 1150ºC developed a lighter pink color with higher luminosity in comparison with those calcined at 1050ºC.
As the concentration of hematite raises, the reflectance percentage decreases, so as the luminosity. The color became more dark for higher chromophorous percentages. This was expected, since the concentration raise increases the absorbed light amount. For the percentages of 10 and 15 wt% chromophorous material, the curves overlapped, indicating proximity of the tonalities obtained for both temperatures.
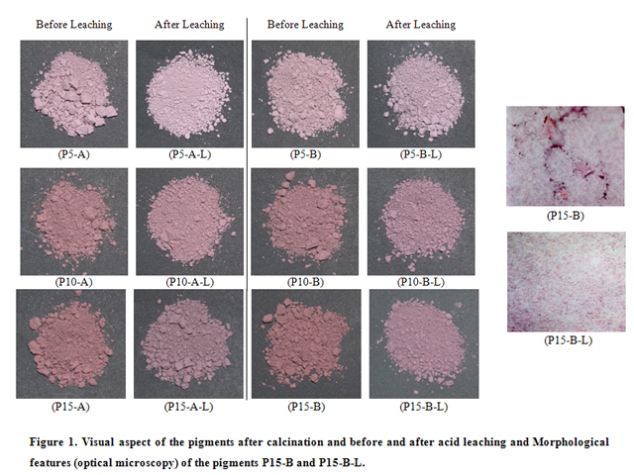
Microscopical techniques were used (optical and SEM) in order to verify the pigment microstructure and the occlusion process in the P15-B and P15-B-L samples. P15-B presented highest chromophorous and lowest non-encapsulated hematite percentage. It can be verified that before acid leaching, P15-B, there is non-encapsulated hematite represented by the red tonality. After leaching, P15-B-L, such agglomerates were not detected any longer. After leaching, there is only silica crystals and encapsulated crystals.
CONCLUSÕES: For all tested conditions the same crystalline phases were obtained and hematite reduction process was not detected. After calcination, the pigments developed a pink tonality with varying saturation and luminosity.
Encapsulating tests showed a high encapsulating efficiency between 92.2 and 98.6%.
The results confirmed the possibility of recycling of rice husk and steel scrap as alternative sources of silica and iron oxide, for obtaining a ceramic pigment by the encapsulating method, turning then these industrial wastes into higher added value products.
AGRADECIMENTOS: - Center of Technology in Materials – CTCMat (Criciúma, SC) (characterization).
- Gerdau - Aços Finos (Piratini, RS) (steel scrap).
- CNPq
REFERÊNCIAS BIBLIOGRÁFICA: 1. Bondioli F, Barbieri L, Manfredini T. Grey ceramic pigment (Fe, Zn) Cr2O4 obtained from industrial dly-ash. Tile & Brick Inter. 2000;16:246-248.
2. Bondioli F, Manfredini T, Pellacani GC. Inorganic pigments for ceramic tiles: Characteristics and industrial applications. Intercer. 1999;48:414-422.
3. Bondioli F, Manfredini T, Siligardi C, Ferrari AM. A new glass-ceramic red pigment. J. Eur. Ceram. Soc. 2004;24:3593-3601.
4. Monrós G, Garcia A, Sorli S, Llusar M, Calbo J. Tena MA. Mecanismos de síntesis de pigmentos heteromórficos. Qualicer 2002:61-75.
5. Società Ceramica Italiana (SCI). Colore, Pigmenti e Colorazione in Ceramica. S.A.L.A. 2003; 360.
6. Lambies V, Rincón JM. Study of the mechanism of formation of a zircon-cadmium sulphoselenide pigment. Trans. Br. Ceram. Soc. 1981;80:105-108.
7. Llusar M, Badenes JA, Calbo J, Tena MA, Morós G. Environmental and colour optimisation of mineraliser addition in synthesis of iron zircon ceramic pigment. Br. Ceram. Trans. 2000;99:14-22.
8. Della VP, Junkes JA, Oliveira APN, Hotza D. Estudo comparativo entre a sílica obtida por lixívia ácida da casca de arroz e a sílica obtida por tratamento térmico da cinza de casca de arroz. Quim. Nova 2006; 29:1175-1179.
9. Junkes JA, Della VP, Acchar W, Oliveira APN, Hotza D. Obtaining amorphous silica from acid-leached calcined rice husk. Ind. Ceram. 2006; 26:11-15.
10. Núñez I, Poré JV, Escribano P, Cordoncillo E, Carda JB. Uma experiencia docente en el estudio de la síntesis y aplicación del pigmento cerâmico Fe-ZrSiO4. Ceram. Inform. 1996; 221:3-18.
