ÁREA: Materiais
TÍTULO: Application of organo-modified attapulgite as adsorbent for uranyl uptake from aqueous solution
AUTORES: SILVA, E. M. (UFMT) ; GUERRA, D. L. (UFMT) ; AIROLDI, C. (UNICAMP)
RESUMO: An attapulgite (ATPG) clay sample has been chemically modified with silylating agent
N-propyldiethylenetrimethoxysilane (NPTM). The resulting matrix has been
characterized by X ray diffractometry and scanning electron microscopy. The natural
and modified clay samples have been tested as potential adsorbents for the removal
of uranyl(II) from aqueous solution at pH 2.0 and 25±1 ºC. The maximum number of
moles adsorbed was determined to be 5.55, and 18.99×10-2 mmol/g for ATPG, and
ATPGNPTM, respectively. Calorimetric determinations gave exothermic enthalpy,
negative Gibbs free energy, and positive entropy. These thermodynamic data confirmed
the energetically favorable condition of such interaction at the solid/liquid the
system.
PALAVRAS CHAVES: attapulgite; surface properties; adsorption
INTRODUÇÃO: A generation of nanoscaled compounds, consisting of inorganic-organic hybrids,
displays a promising class of materials [1-3]. The chemical modification of natural
or synthetic materials is an elaborate task resulting in improvements in the original
surface properties of the materials for academic and technological purposes. These
materials are widely used in reaction catalysis, as adsorbants in aqueous medium, in
the production of nanocomposites, as antibacterial materials, etc [4]. When toxic
metals are present in the aquatic system, the reduction of the pollutants to
acceptable levels is necessary. Adsorption and ion exchange processes are the most
useful methods to remove them. In recent years, special attention has been focused on
the use of natural adsorbants as an alternative to replace the conventional
adsorbants, based on both the environmental and economic points of view [2-4]. This
work reports the use of a chemically modified attapulgite as an alternative for
extracting the toxic ion, uranyl(II), of aqueous solution. The energetic effect
caused by uranyl(II)/basic centers on attapulgite interaction at the solid/liquid
interface was determined through a calorimetric titration procedure.
MATERIAL E MÉTODOS: The natural clay sample was ground and sieved through a USS 200 mesh (0.074mm) sieve,
dried in an oven at 60 ºC to reach humidity between 12% and 15%. A preliminary X-ray
powder diffractometry analysis was used to confirm the presence of the attapulgite
clay fraction. A portion of about 10 g of attapulgite (ATPG) was suspended in 25.0 mL
of DMSO under a nitrogen purge at 25±1 ºC for 1h. Then, 3.0 mL of silylating agent,
N-propyldiethylenetrimethoxysilane (NPTM) was added under flowing nitrogen to the
suspension followed by another 72h at 90±1 ºC. The solid was filtered through
sintered glass, washed with DMSO and again with acetone in a Soxhlet system to remove
the inserting solvent from inside the layers, then dried in vacuum for 12 h, to yield
the immobilized compound denoted ATPGNPTM. X-ray powder diffraction (XRD) patterns
were recorded with a Philips PW 1050 diffractometer using Cu (0.154 nm) radiation in
the region between 2º and 65º at a speed of 2º per min and a step of 0.050º. The
samples for scanning electron microscopy (SEM) images were recorded on a model LEO-
ZEISS, 430 Vp at UNICAMP/Brazil, in conditions of analysis using secondary images
obtained to 20 kV, with a working distance of 11mm. The batch methodology was used to
concentrate uranyl cation from solution in order to obtain the Langmuir isotherm [4].
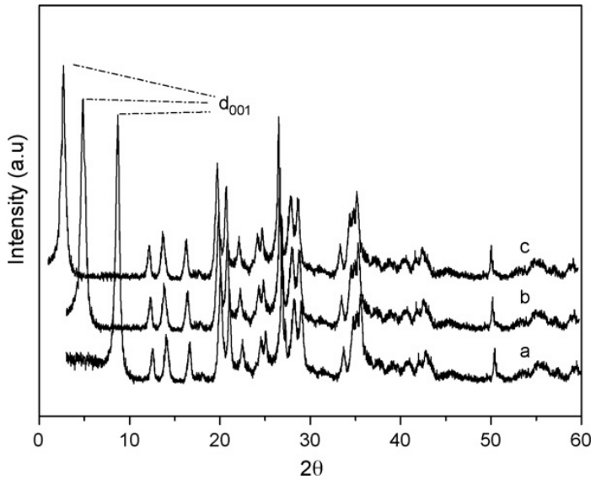
RESULTADOS E DISCUSSÃO: X-ray powder diffraction of ATPG and ATPGNPTM showed significant charges. In Fig. 1
an increase in interlayer distances is clearly observed after intercalation process,
by changing from 1.20 and 3.22 nm for chemically modified clay. As expected, the
silylating agent is covalently bonded inside the free cavity formed by attapulgite
layers, through the reaction with the available hydroxyl groups inside the inorganic
surfaces. Based on the inorganic tetrahedral–octahedral–tetrahedral attapulgite
arrangement with a layer thickness of 0.89 nm, the immobilized pendant chains can be
accommodated along the original 0.10 nm. From these values, net expansions of 2.33 nm
corresponds to the final arrangements after inserting the silylating agents inside
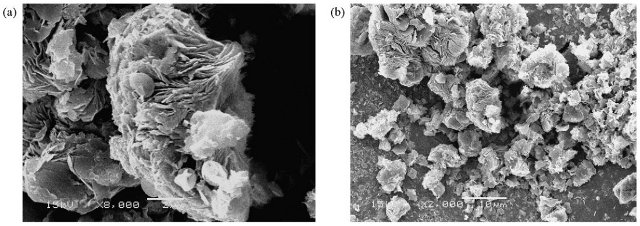
the free cavity space. The SEM images of unmodified (ATPG) and modified (ATPGNPTM)
attapulgite samples are showed in Figs. 2a and b. In the image of natural attapulgite
was observed several agglomerates of irregular shapes. The strong tendency toward
aggregation and the compact aspect of the modified material can also be observed. In
theses cases, the particles are apparently smaller in size and are constituted of
disordered, thin sheet particle aggregates. It can be concluded that
functionalization promotes the formation of disordered and less cohesive aggregates,
probably due to reduction of the edge-to-edge and face-to-face interactions. After
the adsorption tests, a favorable condition was attributed to the available edge
sites on the inorganic backbone, to give the order of affinities of 5.55 and
14.86×10-2 mmol/g, for original attapulgite and those anchored with nitrogen atoms,
respectively.
CONCLUSÕES: A highly stable natural attapulgite, originally from the southeast region of Brazil,
had the surface changed with silylating agents covalently bonded on the inorganic
framework, changing the physical–chemical properties. Chemically modified clay samples
containing nitrogen atoms attached to the pendant chains increased the capacity for
uranyl removal, a favorable condition attributed to the available edge sites on the
inorganic backbone, to give the order of affinities of 5.55 and 14.86×10-2 mmol/g, for
original attapulgite and those anchored with nitrogen atoms, respectively.
AGRADECIMENTOS:
REFERÊNCIAS BIBLIOGRÁFICA: [1]Guerra, D. L., Airoldi, C., Lemos, V.P., Angélica, R.S. and Viana, R.R., 2008, Application of Zr/Ti-PILC in the adsorption process of Cu(II), Co(II) and Ni(II) using adsorption physical chemical models and thermodynamic of process. Química Nova, 31:356–359.
[2]Prado, A.G.S. and Airoldi, C., 2001, Adsorption preconcentration and separation of cations on silica gel chemically modified with the herbicide 2,4-dichlorophenoxyacetic acid. Anal. Chim. Acta, 432:201–211.
[3]Pagana, A.E., Sklari, S.D., Kikkinides, E.S. and Zaspalis, V.T., 2008, Microporous ceramic membrane technology for the removal of arsenic and chromium ions from contaminated water. Micropor. Mesopor. Mater., 110: 150–156.
[4]Guerra, D. L., Silva, E. M., Airoldi, C. 2010, Application of modified attapulgites as adsorbents for uranyl uptake from aqueous solution—Thermodynamic approach. Process Safety and Environmental Protection, 88: 53–61.
