ÁREA: Materiais
TÍTULO: Thermochemical data for n-alkylmonoamines functionalization into tectosilicate heulandite
AUTORES: SILVA, W.L.L. (UFMT) ; OLIVEIRA, S.P. (UFMT) ; GUERRA, D.J.L. (UFMT) ; VIANA, R.R. (UFMT) ; AIROLDI, C. (UNICAMP)
RESUMO: An analogue of heulandite was synthesized by using inorganic salts as a source for silicon and aluminum in the hydrothermal synthesis of the material. The resulting solid was used as hosts for functionalization of polar n-alkylamine molecules of the general formula H3C(CH2)n-NH2 (n = 1 to 4) in aqueous solution. The compound was calorimetrically titrated with amine in ethanol or 1,2-dichloroethane, requiring three independent operations: (i) titration of matrix with amine, (ii) matrix salvation, and (iii) dilution of the amine solution. From those thermal effects the variation in enthalpy was calculated as: -4.51, -5.29, -6.79, and -7.50 kJmol-1, for n = 1 to 4, respectively. The exothermic enthalpic values reflected a favorable energetic process of amine-host functionalization in ethanol.
PALAVRAS CHAVES: heulandite, adsorption, amine.
INTRODUÇÃO: Natural and synthetic zeolites have been tried for the uptake of heavy metals. Zeolite with framework structures is an excellent inorganic ion exchanger. According to this heulandite comes under group 7, heulandite is the name of a series of tectosilicate minerals of the zeolite group ([Ca2,Na4][Al8Si28O72].[24H2O]) (FYFE et al., 2007). Among molecular sieves produced by isomorphous substitution in the silicate structure, zeolites group is the best known example. The isomorphous substitution of aluminum by silicon is the heart of all properties of this class of materials (GUERRA et al., 2008, GUERRA et al., 2009, LAZARIN et al., 2005). The insertion process of organic neutral polar molecules into the nanospace void of sheets of layered insoluble nanocompounds leads to well-organized inorganic/organic structural layer materials (WYPYCH et al., 2003, DIAZ et al., 2007). Advances in the organofunctionalization field have been obtained by observing if any property of the lamellar nanocompound charges, by comparing these with those related to the host, as well as to the inserted guest molecules at the end of the process (DEY et al., 2008; CHAUDHARI et al., 2005; CLEARFIELD, 1990; CAPKOVÁ et al. 2003). Covalent immobilization onto solid supports of a desired chelate moiety, with the specific purpose of obtaining selectively adsorbent materials, is one of the most important procedures to develop highly selective matrices (PRADO et al., 2002; ESPINA et al., 1998; GOUBITZ et al., 2001).The present investigation reports calorimetric determinations involving the functionalization of synthetic heulandite with polar n-alkylamine molecules, CH3(CH2)n-NH2 (n=1-4), in order to study the energetics functionalization.
MATERIAL E MÉTODOS: Reagent grade solvents were used. Amines (Aldrich) of the general formula H3C(CH2)n-NH2 (n=1-4), i.e. ethyl, propyl, butyl, penthylamines and 3-chloropropyltrimethoxysilane (CTS) (Aldrich) were used. Other chemicals such as 1,2-dichloroethane and ethanol were of reagent grade. Doubly distilled deionized water (DDW) was used for the preparation of solutions, wherever required. A sample of 20.0 g of activated heulandite was suspended in 100 cm3 of dry toluene and 20.0 cm3 (108.0 mmol) of CTS was added to this suspension. The mixture was mechanically stirred under reflux of the solvent in an inert atmosphere for 72 h. The suspension was filtered and the solid was washed with toluene and dried under vacuum at 230 K for several hours, to give the modified heulandite, named HEUCL (PRADO et al., 2001). The functionalization was carried out by suspending about 50 mg of synthetic heulandite in 0.50 mol dm-3 solutions of each amine dissolved in ethanol or in 1,2-dichloroethane at room temperature. The n-alkylamine molecules functionalization was performed by the batch method (NUNES et al., 2000; MACHADO et al., 2006). The thermal effects from adsorption reaction were followed by calorimetric titrations using an isothermal calorimeter, Model LKB 2277, from thermometric. The thermodynamic data were fitted employing the nonlinear fitting method using the non-linear fitting facilities of the software Microcal Origin 7.0.
RESULTADOS E DISCUSSÃO: The thermodynamic data related to amine functionalization into the free cavity of the heulandite in ethanol or 1, 2-dichloroethane are listed in Figures 1 and 2, respectively, resulting in an exothermic effect from the net interactive effects. The results showed that an increase in carbon number in the n-alkyl chain induces a corresponding enhancement in the exothermicity of the enthalpy of functionalization. For example, for ethylamine and penthylamine, the values of enthalpy of functionalization are -4.50 and 10.35 kJmol-1, respectively, data which reflects a favorable energetics process of functionalization. The Gibbs free energy values for all functionalizations were negative, indicating that the reactions are spontaneous in nature for both systems. Positive entropic values are also consistent with the arguments that the functionalization process is entropically favored. These values suggest a disoption of the molecules of the solvents originally bonded in the free cavity to the heulandite, as the guest molecules are progressively functionalized.
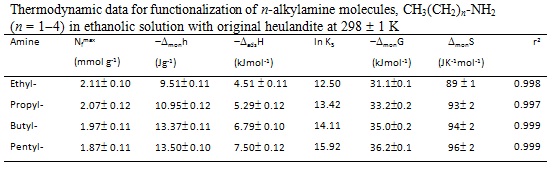
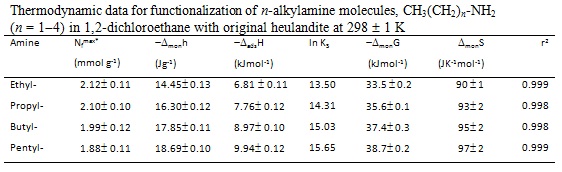
CONCLUSÕES: The tectosilicates derived from the silylating agent and amines: (i) 3-chloropropyltrimethoxysilane and (ii) the reaction product of this precursor with H3C(CH2)n-NH2 (n=1-4), enabled syntheses of well-defined silicate hybrids classified as good host support for organic polar molecules, The functionalization heulandite processes were similar in the two ethanolic medium. Through calorimetric investigation information about all systems was obtained, resulting in exothermic enthalpy, negative Gibbs free energy and positive entropy.
AGRADECIMENTOS:
REFERÊNCIAS BIBLIOGRÁFICA: CAPKOVÁ P., POSPÍSIL M., WEISS Z. 2003. J. Incl. Phenom. 47: 1-10.
CHAUDHARI A., KUMAR C.V. 2005. Micropor. Mesopor. Mater. 77: 175-187.
CLEARFIELD A. 1990. Comm. Inorg. 10: 896.
DEY R., AIROLDI C. 2008. J. Harzard. Mater. 156: 95-101.
DIAZ U., A. CANTIN, A. CORMA. 2007. Chem. Mater. 19: 3686-3693.
ESPINA A., MENÉNDEZ F., JAIMEZ E., KHAINAKOV S.A., TROBAJO C., GARCÍA J.R., RODRIGUES J. 1998. Chem. Mater. 10: 2490-2496.
FYFE C.G., GOBBI G.C., KLINOWSKI J., THOMAS J.M., RAMDAS S. 1982. Nature 296: 530-536.
GOUBITZ K., CAPKOVÁ P., MELÁNOVÁ K., MOLLEMAN W., SCHENK H. 2001. Acta Cryst. B 57: 178-183.
GUERRA D.L., AIROLDI C., VIANA R.R. 2008. Inorg. Chem. Commun. 11: 20-23.
GUERRA D.L., VIANA R.R., AIROLDI C.. 2009. J. Colloid Interface Sci. 337: 122-130.
LAZARIN A.M., LIMA I.S., AIROLDI C. 2005. Solid State Sci. 7: 1423-1428.
MACHADO M.O., LAZARIN A.M., AIROLDI C. 2006. J. chem. Thermodyn 38: 130-135.
NUNES L.M., AIROLDI C. 2000. J. Solid State Chem. 154: 557-563.
PRADO A.G.S., AIROLDI C. 2001. J. Colloid Interface Sci. 236: 161-165.
PRADO A.G.S., ARAKAKI L.N.H., AIROLDI C. 2002. Green Chem. 4: 42-46.
THOMAS J.M. 2007. Micropor. Mesopor. Mater. 104: 5-9.
WYPYCH F., SCHREINER W.H., MATTOSO N., MOSCA D.A., MARANGONI R., BENTO C.A.S.. 2003. MATER J.. CHEM. 13: 304-307.
