ÁREA: Bioquímica e Biotecnologia
TÍTULO: EFFECTS OF DIFFERENT CULTURES MEDIUM ON Dunaliella tertiolecta GROWTH
AUTORES: Neto, C.R.L.A.L. (UNICAP) ; Silva, P.E.C. (UFPE) ; Bezerra, R.P. (UFRPE) ; Marques, D.A.V. (UFRPE) ; Neves, A.L.S. (UFRPE) ; Neto, N.S.O. (UFRPE) ; Carvalho, J.C.M. (USP) ; Porto, A.L.F. (UFRPE)
RESUMO: Dunaliella tertiolecta, a green halophilic microalga, is a potential natural source of highly valuable compounds. Biomass production is limited by several factors, such as nutrients presented in the culture medium that play a key role. In this context, the effects of three culture media were studied on the growing of microalgae Dunaliella tertiolecta. The cultures medium were: (1) Guilard F/2 medium; (2) HEJAZI and WIFJJELS (2003); (3) and WIFJJELS (2003) with replacement of KNO3 by NaNO3. The highest cell concentration, 881 ± 27 mg L-1, was obtained in the Guilard F/2 medium, showing to be the most suitable for Dunaliella tertiolecta microalgae growth.
PALAVRAS CHAVES: Dunaliella tertiolecta; Culture Medium; Cell Growth
INTRODUÇÃO: Dunaliella sp. is the best source of natural β-carotene, which can be used as coloring agents in nutraceuticals, pharmaceuticals, cosmetics and foods. Commercial exploitation of these products needs high cell densities to reduce production costs. Biomass production rate are limited by several factors, such as nutrients presented in the culture medium that play a key role. Guillard F/2 medium (GUILLARD and RYTHER, 1962), HEJAZI and WIJFFELS (2003) medium (CHEN et al., 2011) and Erdschreiber’s medium (CHEN et al., 2011a) are some culture media to Dunaliella sp. cultivation described in the literature. The choice of a culture medium to cell growth is extremely important to guarantee the high cell concentration. The medium should be economic, friendly environmental and allow an excellent growth rate. The complexity and cost of the culture media often excludes their use for large-scale culture operations. Alternative enrichment media that are suitable for mass production of micro-algae in large-scale extensive systems contain only the most essential nutrients and are composed of agriculture-grade rather than laboratory-grade chemical compounds. The objective of this study was evaluated Dunaliella tertiolecta growth under different culture media.
MATERIAL E MÉTODOS: Dunaliella tertiolecta UTEX 999 was cultured in 500 mL flasks with 200 mL of culture medium and initial cell concentration of 50 mg mL−1. The cultures was continuous illuminated at light intensity of 14 μmol photons m-2•s-1 and supplied with air bubbled through an air pump. The temperature of the culture was maintained at 25±2ºC. During the cultivation, cell density was measured daily by spectrophotometer, at the absorbance of 685 nm, and comparing these values with previously prepared calibration curves of optical density versus biomass dry weight.
Three different growth media were used throughout the experiments: one is constituted by natural seawater (1) and other two (2 and 3) are synthetic media chemically defined. All are described above:
(1) Medium culture 1: Guillard F/2 medium constituted by natural seawater and supplemented by salts (GUILLARD and RYTHER, 1962).
(2) Medium culture 2: HEJAZI and WIJFFELS medium (HEJAZI and WIJFFELS (2003) constituted by 2.0 M NaCl, 5 mM KNO3, 0.1mM NaH2PO4.2H2O, 5 mM MgSO4.7H2O, 1 mM KCl, 10 mM NaHCO3, and 0.3mM CaCl2.2H2O. The other materials used were 1 mL/L of trace elements stock with 16.2 mM H3BO3, 9.1mM MnCl2.4H2O, 0.77mM ZnSO4.7H2O, 0.32 mM CuSO4.5H2O, 0.1 mM Na2MoO4, 9.0mM MnSO4.7H2O, and 0.5 mL/L of Fe-salting liquid stock with 0.56 mM Na2EDTA.2H2O, and 0.77 mM FeCl3.6H2O.
(3) Medium culture 3: This medium corresponding to medium culture 2 described by HEJAZI and WIJFFELS (2003), with replacement of nitrogen source KNO3 by NaNO3. This replacement was proposed due a difficulty of buying KNO3, since its sale is controlled by the army, for being a chemical compound used in the production of explosives.
Samples were withdrawn during 21 days to biomass determination. Two replicates were set for each culture condition.
RESULTADOS E DISCUSSÃO: Figure 1 shows that D. tertiolecta was capable to adapt easily in three culture media and that cells multiplication increased in the exponentially growth phase and reached the stationary phase after 18 days when utilized the culture medium (1) and around 15 days, in the culture media (2) and (3). Although the microalgae biomass curve have been shortened in culture media 2 and 3, the growth was reduced compared with the culture medium (1).
The highest cell concentrations were of 881 ± 27 mg L-1(18 days), 495 ± 32 mg L-1 (15 days) and 310 ± 21 mg.L-1 (15 days) in the culture media (1), (3) and (2), respectively. The best result was observed in the culture medium (1) with biomass concentration almost 2.0 and 2.5 higher than culture media (2) and (3), respectively, indicating the culture medium (1) most suitable for the cultivation of D. tertiolecta. Culture media (2) and (3) are chemically defined while the culture medium (1), it be constituted by seawater, can present microelements (BASEGGIO, 1974), that probably improved the D. tertiolecta growth.
Comparing the cultivation on culture media (2) and (3), it was observed that the replacement of KNO3 by NaNO3 increase the cell concentration of 304 ± 21 mg L-1 to 440 ± 32 mg L-1, indicating that the use of NaNO3 improve the cell growth, besides being easier in purchase of the product.
The results obtained in this study demonstrated that the highest cell concentration (881 ± 27 mg L-1) was similar to those obtained by D. salina cultivated in f/2 medium (880 -1150 mg L-1, Huang et al 2011).
Figure 1
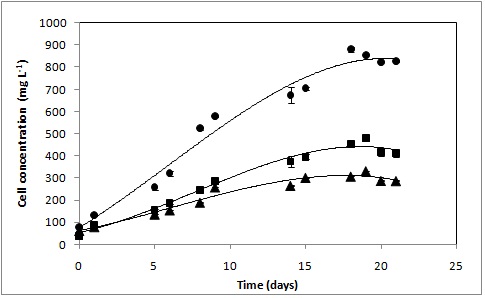
Effects of Guillard F/2(●), Hejazi and Wijffels medium(▲) and Hejazi and Wijffels medium with replacement of KNO3 by NaNO3(■) on D. tertiolecta growth
CONCLUSÕES: The Guilard f/2 medium is better for obtaining high cell density of Dunaliella tertiolecta.
AGRADECIMENTOS: The authors acknowledge the financial support of the FACEPE, CAPES and CNPq
REFERÊNCIAS BIBLIOGRÁFICA: BASEGGIO, G. 1974. The composition of sea water and its concentrates. In: COOGAN, A.H. Ed., Foruth Symposium on salt., vol.2, Northen Geol. Soc., Cleveland, pp. 351-358.
CHEN, H., LAO, Y.M., JIANG, J. G. 2011. Effects of salinities on the gene expression of a (NAD+)-dependent glycerol-3-phosphate dehydrogenase in Dunaliella salina. Science of the Total Environment, 409: 1291–1297a.
CHEN, M., TANG, H.; MA, H.; HOLLAND, T.C: SIMON; K.Y., STEVEN, O., SALLY, O. 2011. Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresource Technology 102:1649–1655
HUAND, W.W., DONG, B.;CAI, Z.; DUAN, S. 2011 Growth effects on mixed culture of Dunaliella salina and Phaeodactylum tricornutum under different inoculation densities and nitrogen concentrations. African Journal of Biotechnology, 10(61):. 13164-1317
GARRIDO, I.M. 2008. Microalgae immobilization: Current techniques and uses. Bioresource Technology. 99(10): 3949-3964.
GUILLARD, R.R. and RYTHER, J.H. 1962. Studies on marine planktonic diatoms. I. Cyclotellananta Hustedt and Detonula confervacea (Cleve) Gran. Can J Microbiol 8: 229–239.
