Antioxidant Capacity of Natives Amazonian Fruits by Nitrotetrazolium Blue Chloride (NBT) method
ISBN 978-85-85905-21-7
Área
Alimentos
Autores
Márcia Becker, M. (UFMA) ; Marques Mandaji, C. (UFMA) ; Gonçalves Lima Neto, L. (CEUMA) ; Badea, M. (TRANSILVANIA UNIVERSITY OF BRASOV) ; Silva Nunes, G. (UFMA) ; Mathaus Ramos Pestana, Y. (UFMA)
Resumo
The increase of oxidative stress drives the search for foods that can prevent or minimize oxidative damage. The antioxidant capacity of four native fruits of the Amazon region was evaluated by NBT method. All the fruits showed antioxidant activity, and cupuaçu and bacuri showed similar statistically by F test and t Student unpaired (p <0.05). The inhibition percentage of radical O2•- was greater for acerola (96.84%) followed by the açaí, murici, cupuaçu/bacuri. This study provided new data on the antioxidant activity of native fruits of the Amazon region, as well as new options for assessing antioxidant capacity of complex matrices. Based on this study, it can be said that these fruits are suitable for use in the food and cosmetics industries as well as in pharmaceutical compositions.
Palavras chaves
Amazonian; antioxidant potential; fruits
Introdução
Amazon Region is formed by a complex mosaic of endemic areas with rich diversity of fruit species which are distributed in accordance with their biota specificities [1]. Recognized sources of nutrients, fruits comprise important foods nutritionally in the human diet and have in recent years received increased attention due to epidemiological evidence that shows that the regular consumption of vegetables reduces mortality and morbidity due to some chronic diseases [2, 3]. The protective effect exerted by these foods has been attributed to high levels of phytochemicals with antioxidant properties that contribute to the prevention of various diseases [4]. Antioxidants are natural or synthetic substances that prevent or delay the oxidative damage by scavenging the free radicals, even in small concentrations, being formed by enzymes, vitamins, minerals, phenolic compounds, flavonoids and protein [5, 6]. Although some antioxidant molecules have biological origin, the daily intake of antioxidants through diet is necessary in view of its constant physiological consumption for maintaining the balance of free radicals. Once in healthy individuals the production of free radicals is balanced by the antioxidative defense system, the imbalance in favor of free radicals generates the oxidative stress that may be a contributory factor to the pathogenesis such as neurodegenerative disorders, Alzheimer's diseases, Parkinson's diseases, cancer, cardiovascular diseases, atherosclerosis, cataracts, cognitive dysfunction and inflammations [7-10]. Free radicals are highly unstable molecules oxidation with available electrons that can be generated in vivo during metabolic processes and/or by exogenous sources, as some carcinogenic compounds and ionizant reactions. The Reactive Oxygen Species (ROS) are the most damage class of free radicals in biological systems, and include the superoxide anion (O2•-), singlet oxygen (O21), hydrogen peroxide (H2O2), hypochlorous acid (HOCl), hydroxyl radical (OH•), peroxynitrite (ONOO-), peroxyl (ROO•), alkoxyl radicals (RO•) and others [11]. Thus, it is important to know the antioxidant content and their efficacy in fruits, for preservation or protection against oxidative damage, as well as for future cosmetic, therapeutic, medical and food technology applications. There are many spectrophotometric methods for measuring the antioxidants potential in vegetables. In general, are simple and rapid assays and needs only a UV-vis spectrophotometer. But the most measure the ability to inhibit a commercial free radical, that is, a free radical with chemical structures other than free radicals produced in biological systems. In this view, the NBT method shows advantages, once evaluate the superoxide scavenging capacity of the antioxidants in the sample, in physiological conditions. Established by Cortina Puig et al. (2009), in the NBT method, O2•– radicals and acid uric are generate in vitro by the HX/XOD system. The O2•– radicals reduce the NBT reagent (yellow color) into formazan (purple color), which is measure spectrophotometrically at 560 nm (Figure 1). The presence of radical scavengers (the antioxidant sample) generates inhibition (competitive) in the formation of formazan leading to the decrease of its production rate. Considering the unexplored antioxidant potential of fruits native to the Amazon region, this paper determined the antioxidant capacity by colorimetric assay for four Amazonian fruits, popularly known as açaí (Euterpe oleracea), bacuri (Platonia insignis), cupuaçu (Theobroma grandiflorum) and murici (Byrsonima dealbata). For this, the production of oxygen radicals from hypoxanthine system (HX) / xanthine oxidase (XOD) was carried out in the presence of nitrotetrazolium blue chloride (NBT), allowing the formation of a product with a linear purple color and stable which is measured spectrophotometrically at 560 nm [12]. Before the unexplored antioxidant potential of fruits native to the Amazon region, this paper determined the antioxidant capacity by colorimetric assay for four Amazonian fruits, popularly known as açaí (Euterpe oleracea), bacuri (Platonia insignis), cupuaçu (Theobroma grandiflorum) and murici (Byrsonima dealbata). For this, the production of oxygen radicals from hypoxanthine system (HX) / xanthine oxidase (XOD) was carried out in the presence of nitrotetrazolium blue chloride (NBT), allowing the formation of a product with a linear purple color and stable which is measured spectrophotometrically at 560 nm [12].
Material e métodos
CHEMICAL REAGENTS The reagents used were NBT (N6876), HX (H9377), L-ascorbic acid (A5960), XOD from bovine milk (X4376). All reagents were of analytical grade and all solutions were prepared using Milli-Q water. INSTRUMENTATION Colorimetric measurements were performed with a Beckman DU520 UV–Vis Spectrophotometer (Beckman Coulter France, S.A., Roissy CDG, France). PREPARATION OF THE ANTIOXIDANT SAMPLES Four native amazonic fruits were included in this study: açaí (Euterpe oleracea), bacuri (Platonia insignis), cupuaçu (Theobroma grandiflorum) and murici (Byrsonima dealbata). The fruits were obtained from São Luís, Maranhão, Brazil in Ceasa market. Acerola was also evaluated in this study in order to compare their results with other fruit, in view because it is a known high antioxidant potential [13, 14]. The samples were washed, pulped and mixed with the aid of a mixer of stainless steel. Approximately, and triplicate samples of 200 g were taken, filtered in vacuum Buchner funnel with qualitative filter paper, followed by a new filtration with a quantitative filter paper (1.2 mM). The volume of 500 uL of the obtained extract was diluted to 1 mL in 50 mM Phosphate-Buffered Potassium (K-PB) pH 7.5 with 0.1 mM EDTA. From this solution, sequential dilutions were performed using 50 mM Phosphate-Buffered Potassium (K-PB) pH 7.5 with 0.1 mM EDTA. MEASUREMENT OF THE SUPEROXIDE SCAVENGING CAPACITY USING THE NBT METHOD A reaction mixture was prepared with 50 mM K-PB pH 7.5 containing EDTA (0.1 mM), 25 μM HX, 50 μM NBT, the antioxidant extract (distilled water for the blank) and 0.2 U mL−1 XOD, which was added last. The increase in absorbance for 3 min was recorded at 560 nm. Stock solutions of NBT, HX and XOD were prepared in 50 mM K-PB pH 7.5 containing EDTA (0.1 mM). The % radical scavenging activity (RSA) of the plant extracts was calculated using the following formula: RSA % = 100 x [(Abs. control - Abs. sample)/Abs control] Where, Abs. control is the absorbance of formazan without the sample; Abs. sample is the absorbance of formazan with the sample.
Resultado e discussão
Results of the antioxidant activity of fruits under study expressed in function
de formazan production rate for different title mass (% m/v) of analyzed fruits
and standard deviation for each analysis are shown in the Figure 2. There was
observed that all the fruits showed antioxidant activity.
The inhibition of O2•– radicals generated by the antioxidant action of
the studied pulps are revealed by the small NBT amount reduced to formazan.
Evaluating the closeness of the results obtained for bacuri and cupuaçu samples,
F and unpaired t tests were applied using 95 % confidence limits to see if they
show significant differences between their accuracy and absorbance averages,
respectively. Table 1 shows the F and unpaired t values calculated.
The results of the F and unpaired t tests showed that there aren't significant
differences between the precision and the percentage of formazan production
obtained in samples of cupuaçu and bacuri, for all dilutions, at 95 %
confidence, revealing antioxidant activities similar to those plant species.
The results shows the superoxide RSA was biggest in acerola (96.39 %), and lower
for the following fruits by açaí (84.21 %), murici (83.91 %), cupuaçu (80.54 %)
and bacuri (78.34 %).
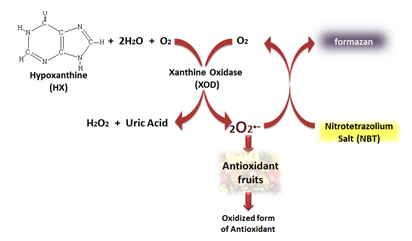
Reactions involved in the measurement of the superoxide scavenging capacity using the NBT chromogenic reagent.

Representation of the formazan production rate by different concentrations of pulp fruits.
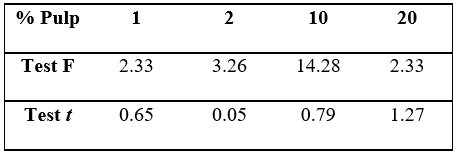
F critical at 95 % confidence for 2 degrees of freedom: 19.0; t critical at 95 % confidence for 4 degrees of freedom: 2.78.
Conclusões
The NBT method employed shown an alternative for the determination of antioxidant capacity in fruits, being promising for application in other matrices, in the same conditions. The addition of fruits pulp samples caused the decrease in absorbance signal, allowing the quantification of their antioxidant capacity. The results reveal that all the fruit studies showed antioxidant activity. The acerola has the highest antioxidant activity, followed by açaí, murici and cupuaçu/bacuri. Cupuaçu and bacuri have similar antioxidant activity for all dilutions made, with 95 % confidence level. Overall, this study provides data on the antioxidant activity of native fruits of the Amazon Region.
Agradecimentos
G. S. Nunes would thank the Fundação de Amparo ao Desenvolvimento Científico e Tecnológico do Maranhão, FAPEMA (Contract. 37906/2016) by the research Grant during thi
Referências
[1] Silva, J. M. C.; Rylands, A. B.; Fonseca, G. A. B. The fate of the Amazonian areas of endemism. Conservation Biology. 2005,19,3, 689-694. [CrossRef]
[2] Rufino, M. S.; Alves, R. E.; Brito, E. S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 nontraditional tropical fruits from Brazil. Food Chemistry. 2010, 121, 996-1002. [CrossRef]
[3] Alissa, E. M.; Ferns, G. A. Functional foods and nutraceuticals in the primary prevention of cardiovascular diseases. Journal of nutrition and metabolism. 2012, 2012. [CrossRef]
[4] Wang, H.; Cao, G.; Prior, R. L. Total antioxidant capacity of fruits. J. Agric. Food Chem. 1996, 44, 3, 701-705. [CrossRef]
[5] Wang, L.F.; Chen, J.Y.; Xie, H.H.; Ju, X.R.; Liu, R.H. Phytochemical profiles and antioxidant activity of adlay varieties. J. Agric. Food Chem. 2013, 61, 5103–5113. [CrossRef]
[6] Halliwell, B. Biochemistry of Oxidative Stress. Biochemical Society Transactions. 2007, 35, 5, 1147-1150. [CrossRef]
[7] Lefer, D. J.; Granger, D.N. Oxidative stress and cardiac disease. Am. J. Med. 2000, 109, 315–323. [CrossRef]
[8] Spector, A. Oxidative stress-induced cataract: mechanism of action. Faseb J. 1995, 9 (12), 1173–1182. [Link]
[9] Shukitt-Hale, B. The effects of aging and oxidative stress on psychomotor and cognitive behavior. Age 22. 1999, 9–17. [CrossRef]
[10] Dreher, D.; Junod, A. F. Role of oxygen free radicals in cancer development. Eur. J. Cancer. 1996, 32, 30–38. [CrossRef]
[11] Halliwell, B.; Aeschbach, R.; Loliger, J.; Aruoma, O.I. The characterization of antioxidants. Food Chem. 1995, 33, 601–617. [CrossRef]
[12] Cortina-Puig, M.; Muñoz-Berbel, X.; Rouillon, R.; Calas-Blanchard, C.; Marty, J. L. Development of a cytochrome c-based screen-printed biosensor for the determination of the antioxidant capacity of orange juices. Bioelectrochemistry. 2009, 76, 76–80. [CrossRef]
[13] Nunes, R. S.; Kahl, V. F.; Sarmento, M. S.; Richter, M. F.; Costa-Lotufo, L. V.; Rodrigues, F. A.; Abin-Carriquiry, J. A.; Martinez, M. M.; Ferronatto, S.; Ferraz, A. De B.; Da Silva, J. Antigenotoxicity and antioxidant activity of Acerola fruit (Malpighia glabra L.) at two stages of ripeness. Plant Foods Hum Nutr. 2011, 66, 129-35. [CrossRef]
[14] Lima, V. L. A. G.; Melo, E. A.; Pinheiro, I. O.; Guerra, N. B. Antioxidant capacity of anthocyanins from acerola genotypes. Capacidade antioxidante de antocianinas de genótipos de acerola. Ciênc. Tecnol. Aliment. 2011, 31 (1), 86-92. [CrossRef]
































