Autores
Gonzalez, R. (UNIVERSIDAD DE TALCA) ; Prent Peñalosa, L. (UNIVERSIDAD ANDRES BELLO) ; Gutierrez, M. (UNIVERSIDAD DE TALCA) ; F. de La Torre, A. (UNIVERSIDAD DE CONCEPCIÓN) ; Concepción, O. (UNIVERSIDAD DE CONCEPCIÓN) ; Caballero, J. (CBSM, UNIVERSIDAD DE TALCA)
Resumo
Sunscreen protect the skin from the sunlight ultraviolet rays that reach the earth (UVA and UVB rays specifically). The UVA and UVB rays can provoke chronic effects such as photocarcinogenesis and photoaging because they penetrate deep into the skin and induce DNA damage, ROS generation, apoptosis, etc. However, developing new organic protectors is a matter of great importance in medical research and the cosmetic industry. The current work provides a multicomponent approach to synthesizing novel molecules with the potential to scatter UVA and UVB rays. Through U-4CR and P-3CR, we obtained in high yield eight α,β–unsaturated carbonyl pseudopeptides mimicking some commercial sunscreen. Also, its photophysical properties were determined using UV and PL techniques.
Palavras chaves
multicomponent reactions; UV blockers; photophysical properties
Introdução
Terrestrial solar ultraviolet (UV) radiation (~295-400 nm) comprises UVA (320-400 nm) and UVB (280-320 nm); prolonged exposure could be mainly harmful to health, and the damage caused can be acute or chronic. Acute effects caused by UVB radiation include erythema, pigmentation, and suppression of acquired immunity, while UVA causes reduction in blood pressure. Chronic effects include photocarcinogenesis and photoaging (YOUNG et al., 2016). Due to this, there are different ways to protect ourselves from the adverse effects of sunlight exposure, one of which is sunscreens applied on the skin.
Different compounds were developed as sunscreen (PANDIKA et al., 2018), classified as physical blockers (inorganic compounds) or as chemical absorbers (organic compounds). Inorganic compounds (TiO2, ZnO) have been employed to scatter UVA and UVB rays, which act as mirrors that deflect the UV rays. These compounds have a high photoprotective capacity and great photostability, but they are not the most widely used, due to adverse cosmetic effects (KOLLIAS, 1999; DRANSFIELD, 2000; ANTONIOU et al. 2008). Organic compounds usually have a conjugated Electron-Withdrawing Group (EWG), such as the carbonyl group attached to a π conjugated group in their structure (e.i., aminobenzoates, cinnamates, salicylates, octocrylene). Thus, these molecules absorb the UV rays and release them as lower-energy rays, less toxic to the skin (SHAATH, 2010; YOUNG et al., 2016).
We are interested in the incursion of the synthesis and discovery of novel photoprotective of UVA and UVB radiation. We used two multicomponent reactions (P–3CR and U–4CR) to obtain α,β–unsaturated carbonyl depsipeptides, and peptoids capable of acting as UVA and UVB blockers, for the cosmetic sector.
Material e métodos
Materials and reagents were of the highest commercially available grade and used without further purification. The melting points (uncorrected) were determined using a Fisher-Johns melting point apparatus (Bibby Scientific Limited, Staffordshire, UK). ATR-FTIR spectra were recorded by using UATR two (Beaconsfield, Bucks, UK) in the range of 4000-400 cm-1 with 16 scans. 1H NMR and 13C NMR spectra were recorded at 400 MHz for 1H and 100 MHz for 13C, respectively using CDCl3 as solvent. Chemical shifts (δ) are reported in ppm relative to the residual solvent signals, and coupling constants (J) are reported in Hz. High–resolution mass spectra (HRMS) were obtained from Thermo Fisher Scientific Exactive Plus mass spectrometer. The analysis was performed at 50 °C; sheath gas flow, 5; sweep gas flow rate, 0 and spray voltage, 3.0 kV at negative mode. Experimental UV spectra were recorded in a Spectroquant® Pharo 300, from 190 nm to 600 nm every 1 nm for each compound in five solvents (Hex, DCM, THF, EtOH and MeCN). The photostability studies were done as described in the ICH HARMONIZED TRIPARTITE GUIDELINE (Q1B).
Depsipeptides were prepared according to this procedure: the p-formaldehyde (1.0 mmol), the respective carboxylic acid (1.0 mmol) and the ciclohexylisocyanide (1.0 mmol) were stirred in DCM (5 mL) for P1-P3 or THF:MeOH (1:1 v/v; 5 mL) for P4 at 25 °C for 24 h. The crude was treated and purified using flash column chromatography (FCC).
Peptoids were prepared according to this procedure. A solution of the benzylamine (1.0 mmol) and p-formaldehyde (1.0 mmol) in MeOH (5 mL) was stirred at 25 °C for 1 h. Then the respective carboxylic acid (1.0 mmol) and the ciclohexylisocyanide (1.0 mmol) was added and left to react for 24 h. The crude was treated and purified using FCC.
Resultado e discussão
Multicomponent reactions (MCR) with isocyanides have become a fundamental tool in developing libraries of compounds with potential uses in pharmaceutical and biomedical studies. By P–3CR, 4 depsipeptides were synthesized, (P1–P4 Fig. 1), reaching between 15 and 40% yields. The low yield values are associated with the poor solubility of the reagents, it should be noted that even so they are comparable with the yields reported, and in agreement with the proposed mechanism of this reaction (REZA et al., 2012; WAHBY et al. 2022). By NMR, the products showed the signals corresponding to the formation of the new bonds. The δ of carbon from the ester function formed at values between 164 and 167 ppm, the amide carbonyls included in the same region, and the unshielded carbon (~63 ppm) and the singlet signals of the methylene protons between carbonyls groups (~4.5 ppm). The spectrophotometric determination demonstrated the strong absorption that all the compounds show in the UV region between 200 and 350 nm (Fig. 1). A total of 4 peptoids were synthesized through the U–4CR (U1–U4), in order to generate the desired electronic delocalization and maintain photophysical properties. The yields achieved above 55% may be due to the improved solubility of the starting acids, and formation of the iminium ion, a more reactive intermediate. The NMR and the other technics allowed for assigning the desired products. Two peptoids showed strong absorption bands at longer wavelengths U2 (287 nm) and U4 (295 nm) with a high-intensity band between 250 and 350 nm (Fig. 2), and showed a negative solvatochromic effect, results comparable with the UV spectrum of avobenzone and its behavior in different solvents (MTURI et al. 2008). In addition, U4 proved to be stable to UV exposure.
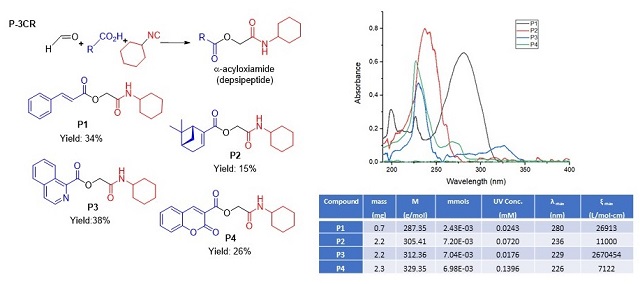
Compounds P1–P4 synthesized by P-3CR, UV spectrum and results in DCM.
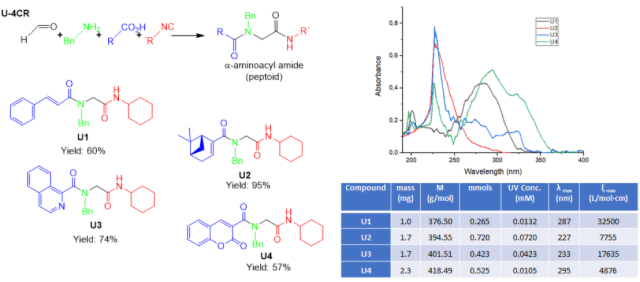
Compounds U1–U4 synthesized by U-4CR, UV spectrum and results in DCM.
Conclusões
In summary, novel depsipeptides and peptoids were obtained with a good yield using one–pot multicomponent reactions (P–3CR and U–4CR). The structural characteristics and photophysical properties of the synthesized molecules allow them to experiment with different processes of electronic delocalization from the π orbitals of the bonds formed and the aromatic rings involved (electronic transitions n π* and π π*), been more interesting in peptoids U1 and U4, with broad absorption bands involving the UVB and UVA region. These compounds can be considered as a potential broad-spectrum sunscreen.
Agradecimentos
M,G acknowledge to FONDECYT project number 1200531, R,G acknowledge to scholarship ANID number 21191215, O,C acknowledge to FONDECYT project No. 3170012, and Fondequip Grants EQM170172
Referências
ANTONIOU, C.; KOSMADAKI, M.; STRATIGOS, A.; KATSAMBAS, A. Sunscreens - What’s Important to Know. Journal of the European Academy of Dermatology and Venereology, v.22, n.9, p.1110–1119, 2008. https://doi.org/10.1111/j.1468-3083.2007.02580.x.
DRANSFIELD, G. P. Inorganic Sunscreens. Radiation Protection Dosimetry, v.91, n.1, p.271–273, 2000. https://doi.org/10.1093/oxfordjournals.rpd.a033216.
KOLLIAS, N. The Absorption Properties of “Physical” Sunscreens. Archives of Dermatological Research, v.135, n.2, p.209–210. 1999. https://doi.org/10.1001/archderm.135.2.209-a.
MTURI, G. J.; MARTINCINGH, B. S. Photostability of the Sunscreening Agent 4-Tert-Butyl-4′-Methoxydibenzoylmethane (Avobenzone) in Solvents of Different Polarity and Proticity. Journal of Photochemistry and Photobiology A: Chemistry, v.200 n.2–3, p.410–420, 2008. https://doi.org/10.1016/j.jphotochem.2008.09.007.
PANDIKA, M. Looking to Nature for New Sunscreens. ACS Central Science, v.4, n.7, p.788–790, 2018. https://doi.org/10.1021/acscentsci.8b00433.
REZA, K. A.; RAMAZANI, A. Synthetic Applications of Passerini Reaction. Current Organic Chemistry, v.16, n.4, p.418–450, 2012. https://doi.org/10.2174/138527212799499868.
SHAATH, N. A. Ultraviolet Filters. Photochemical & Photobiological Sciences, v.9 n.4, p.464, 2010. https://doi.org/10.1039/b9pp00174c.
WAHBY, Y.; ABDEL-HAMID, H.; AYOUP, M. S. Two Decades of Recent Advances of Passerini Reactions: Synthetic and Potential Pharmaceutical Applications. New Journal of Chemistry, v.46, n.4, p.1445–1468, 2022. https://doi.org/10.1039/D1NJ03832J.
YOUNG, A. R.; CLAVEAU, J.; ROSSI, A. B. Ultraviolet Radiation and the Skin: Photobiology and Sunscreen Photoprotection. Journal of the American Academy of Dermatology, v.76, n.3, p.S100–S109, 2017. https://doi.org/10.1016/j.jaad.2016.09.038.
















