Autores
Marques, B.S. (UNIVERSIDADE FEDERAL FLUMINENSE) ; de Souza, M.C. (UNIVERSIDADE FEDERAL FLUMINENSE) ; Pedrosa, L.F. (UNIVERSIDADE FEDERAL FLUMINENSE) ; Botelho, A.B.M. (UNIVERSIDADE FEDERAL FLUMINENSE)
Resumo
In the last decade, fluorescent chromophores BODIPY have received much attention
because of their photophysical and photochemical properties. The range of
applications of this class of compounds can be increased with the introduction of
groups that promote hydrophilicity or amphiphilicity, thus, it could be explored
in sectors such as light-emitting devices, solar cells and chemical sensors. In
this context, the purpose of this work is the development of a new class of
molecular sensors that bring together, in the same molecule, BODIPY (fluorescence)
and organophosphorus (hydrophilicity). To conjugate these blocks, 1,3,5-triazine
was chosen, due to its great convenience in adding up to three interest groups by
replacement of the three chlorine atoms of cyanuric chlorid.
Palavras chaves
BODIPY; Organophosporus; Molecular sensors
Introdução
4,4-difluoro-4-bora-3a,4a-diaza-s-indacene chromophores, better known as
difluoroborodipyrromethenes or, BODIPY, have particular photophysical and
photochemical characteristics, which include strong absorption and emission near
500 nm, high molecular absorption coefficient, relatively long lifetime in the
excited state and excellent photostability. Furthermore, its spectroscopic
properties can be finely tuned by incorporating electron-rich or electron-poor
substituents at appropriate positions on the nucleus. As a result, BODIPY dyes
are found with applications in the areas of biological marking, luminescent
equipment and chemical sensors. (Das et al, 2018; Hall et al, 2019)
Phosphoramidate esters were the organophosphorus functions chosen to integrate
the final molecules of this project, as they stand out for having stable P-N
bonds, for having chelating and photoelectronic properties, and for being
susceptible to hydrolysis under conditions that are not aggressive to the rest
of the molecule. Additionally, the P=O group plays an important role as an
acceptor in hydrogen bonds, considerably increasing the solubility in water, in
addition to favoring the penetration of the compound through biological
membranes. (Koshevoy et al, 2021)
To obtain the fluorescent triads proposed in this project, the central nucleus
1,3,5-triazine was chosen. The experimental conditions for the introduction of
nucleophiles are well known and occur sequentially as a function of temperature:
~0 oC; ~25°C; ~65 oC, respectively. In a recent publication, we developed an
efficient methodology for the synthesis of two new triazine triads coupled to
the BODIPY and porphyrin nuclei, which demonstrated adequate energy transfer for
use in imaging (Souza et al, 2021)
Material e métodos
The sequential substitutions of the three chlorine atoms of cyanuric chloride by
nucleophilic reagents are carried out with control of reaction temperatures
using polar solvents, such as acetone and THF and in the presence of an HCl
acceptor base. Potassium, sodium and cesium carbonates are the most used bases
for oxygen nucleophiles, and diisopropylethylamine (DIPEA) is more used when
amines are nucleophilic.
This project includes the study of the reactivity of three nucleophiles, with
different nucleophilic atoms (O and N), to promote substitutions in cyanuric
chloride: the phenolic BODIPY fluorophore, the water-soluble diisopropyl
aminoalkyl phosphoramidates (n=2.4 ,6) and the (2-aminoethyl)-pyridine
coordination auxiliary group. The final products will be 1,3,5-triazine central
core triads containing at least one BODIPY subunit and other combinations of the
three subunits, representing new compounds with their own characteristics to act
as fluorescent biological sensors.
Resultado e discussão
BODIPY I was synthesized in 29% yield starting from p-hydroxybenzaldehyde and
2,4-dimethyl-pyrrole. Phosphoramidates II were obtained following direct diamine
phosphorylation methodology developed by our research group in a range of 47-52%
yield. After obtaining the nucleophilic intermediates, we faced difficulties in
introducing the BODIPY and phosphoramidate groups. To circumvent this situation,
the aldehyde intermediate V was synthesized in 58% yield, with the sequential
substitution of the (2-aminoethyl)-pyridine III group to obtain the unpublished
intermediate VI, in 81% yield, after purification in a chromatography column
using ethyl acetate and hexane in 1:1 ratio as eluent and recrystallization
using the same solvent. Next, the unpublished BODIPY VII was obtained in 17%
yield by reacting 3 with two equivalents of 2,4-dimethyl-pyrrole under acid
catalysis with TFA, oxidation with p-chloranil, subsequent complexation with
BF3.OEt2, and purification by column chromatography using gradient elution with
hexane and ethyl acetate as eluent. The synthesis of the series of compounds
that make up the triads IV of interest to the project is in the purification
optimization stage, with positive preliminary results. (Figure 1)
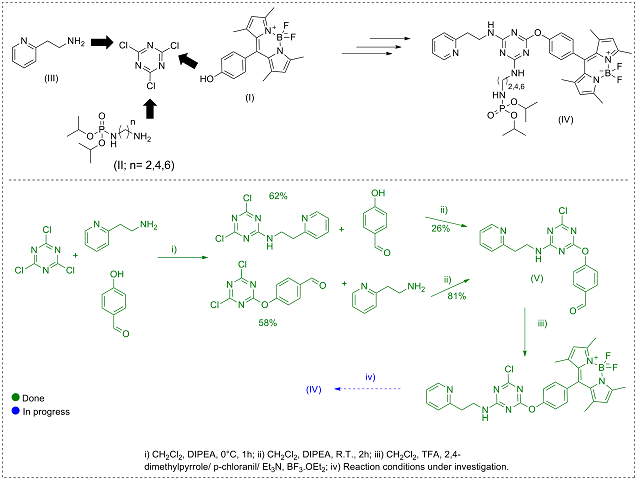
Synthetic pathway planned to obtain the intermediates and the final triads of interest.
Conclusões
In the present work we synthesized all the planned nucleophilic intermediates,
developed a suitable synthetic pathway to introduce the first and second
nucleophiles in the cyanuric chloride, hence, we got two unpublished precursors of
the final products. The next step consists of finalizing the purification of the
final triad and its photophysical and photochemical characterization.
Agradecimentos
To Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ) and
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)- Programa de
Excelência Acadêmica (PROEX) - Brasil for Financial Support.
Referências
DAS, A.; THOMAS, J. A.; SMYTHE, C. G. Tracking HOCl concentrations across cellular organelles in real time using a super resolution microscopy probe. Chem. Commun., v.54, 1849-1852, 2018.
HALL, M. J., CLARKE, R. G. Recent developments in the synthesis of the BODIPY dyes. Advances in Heterocyclic Chemistry, v.128, 181-261, 2019.
KOSHEVOY, I. O, et Al. Cationic Organophosphorus Chromophores: A Diamond in the rough among Ionic Dyes. Chem. Eur. J., v.27, 537-552, 2021.
SOUZA, M. C., SANTOS, C. I. M., MARIZ, I.; et Al. New triazine bridged triads based on BODIPY-porphyrin systems: Extended absorption, efficient energy transfer and upconverted emission. Dyes and Pigments, 2021.
















