Autores
França, S.B. (UNIVERSIDADE FEDERAL DE ALAGOAS) ; Correia, P.R.S. (UNIVERSIDADE FEDERAL DE ALAGOAS) ; Rodrigues, J.E.S. (UNIVERSIDADE FEDERAL DE ALAGOAS) ; Cunha, C.R.S. (UNIVERSIDADE FEDERAL DE ALAGOAS) ; da Silva, D.P. (UNIVERSIDADE FEDERAL DE ALAGOAS) ; Calumby, R.J.N. (UNIVERSIDADE FEDERAL DE ALAGOAS) ; da Silva, V.D. (UNIVERSIDADE FEDERAL DE ALAGOAS) ; Bastos, M.L.A. (UNIVERSIDADE FEDERAL DE ALAGOAS) ; da Silva-junior, E.F. (UNIVERSIDADE FEDERAL DE ALAGOAS) ; Lima, D.J.P. (UNIVERSIDADE FEDERAL DE ALAGOAS)
Resumo
α-Acyloxycarboxamides have multiple biological activities. In view of this, the present work presents a synthesis of seven α-acyloxycarboxamides derived from cinnamic acid and their assessment as larvicidal and antibacterial agents. It is noteworthy that these classes of synthetic compounds, which are novel in the literature, were developed by three steps: HWE olefination reaction, hydrolysis and the Passerini reaction, respectively, with yields ranging from 16 to 59%. They were tested for preliminary larvicidal response against Ae. aegypti larvae and antibacterial evaluation against gram-positive/negative bacteria. Albeit having no larvicidal or antibacterial activities, the synthetic approach developed for this chemical library showed satisfactory outcomes regarding the literature data.
Palavras chaves
Mosquito; Synthesis; Biological active
Introdução
The α-acyloxycarboxamides (or depsipeptides) represent an organic compound class having diversified chemical structures, as they bear varied groups, such as esters, amides, stereogenic centres and n-substituted carbon chains that further enhance their biological activity (HAMADA & SHIOIRI, 2005; AYOUP et al., 2019). Such a class of compounds has a wide range of biological applications, which include anti-microbial, anti-tumor and herbicidal activities (AYOUP et al., 2019; ZONG et al., 2019; ŻELECHOWSKI et al., 2021).
Current research has shown that cyclic depsipeptides are promising antibiotic candidates against certain resistant pathogens (ESKAPE), notably Teixobactin, which displayed outstanding activity against a variety of Gram-positive microorganisms (Staphylococcus aureus and Enterococcus falcium) resistant to methicillin and vancomycin, respectively (JIN et al., 2016).
Additionally, the insecticidal activity has also been found in cyclic depsipeptides, such as the destruxin, produced by entomopathogenic fungi (Metarhizium anisopliae). Its insecticide action was confirmed against Lepidoptera, especially Spodoptera lituca whose larvae displayed tetanus muscle paralysis, followed by flaccidness.
An additional class of chemicals to be highlighted are cinnamic acid derivatives, stemming from the biosynthetic pathway of shikimic acid. This compound library has a range of therapeutic properties, such as anti-inflammatory, anti-oxidant, anti-viral, anti-bacterial, anti-fungal, and anti-cancer activities, drawing the scientific community's attention over the last years (GUNIA-KRZYŻAK et al., 2018; PONTIKI et al., 2014; JITAREANU et al., 2013; MARTÍNEZ-SORIANO et al., 2015; IMAI et al., 2019).
Those compounds exhibit multiple mechanistic and biological actions in living organisms, which are associated with aliphatic side chain modifications or substituent group changes in the ortho, meta, and/or para positions of the aromatic ring of the cinnamoyl moiety. One example of this cinnamic series is the Ethyl p-chlorocinnamate, whose larvicidal activity against Ae. aegypti mosquito larvae was shown to be the most effective (FRANÇA et al., 2021). Therefore, it is observed that the cinnamoyl moiety also plays a vital therapeutic role for the cinnamic acid derivatives, whereas the amide group and the stereogenic center are pivotal for the activity performed by the depsipepitides (AYOUP et al., 2019).
Within this context, it is worth mentioning that both those both chemotherapeutics class are naturally found in low amount, making it unfeasible to employ them on an industrial scale (SAITO &LUCCHINI, 1997; SAURABH et al., 2015). Nevertheless, with the combined organic synthesis and medicinal chemistry these compounds can be produced in reasonable quantities, thereby expanding the industrial market. In this regard, several synthetic routes towards cinnamic acid derivatives have been reported in the literature, including the Perkin, Knoevenagel, Horner-Wadsworth-Emmons, Heck reactions, amongst others. Whilst the Passerini multicomponent reaction is being widely employed for α-acyloxycarboxamides, where it relies on isocyanide chemistry, assisting in bond formation that gives rise to certain pharmacophore groups, with high degrees of chemo-, regio-, and stereoselectivity (KAZEMIZADEH & RAMAZANI, 2009; ALVES, 2014; ROGERIO et al., 2016).
The multi-component reactions (MCRs) are an optimal synthetic strategy towards the synthesis of drug candidate molecules, such as depsipeptides, having simple to complex frameworks, with a certain flexibility, as it allows the formation of diverse functional groups with a wide range of biological activities (ALVES, 2014). Moreover, such reactions are regarded as an ideal green and medicinal chemistry synthesis, due to the fact that they require reduced synthetic steps, provide higher atomic efficiency (economic factor) and generate minimal residues (ROGERIO et al., 2016).
Currently, the Passerini reaction has usefulness in the total synthesis of biologically outstanding natural products, being widely employed in synthetic and medicinal chemistry. A classical method in this reaction is based on polar aprotic solvents, such as ethyl acetate (EtOAc), dichloromethane (CH2CL2), dimethylformamide (DMF) and others, under mild conditions (room temperature and free of inert atmosphere and pressure build-up), resulting in depsipeptides with reasonable yields, although no α-acyloxycarboxamides derived from cinnamic acid have been reported in the literature with satisfactory yields (KAZEMIZADEH & RAMAZANI, 2009; ROGERIO et al., 2016).
Hence, considering the potential of the depsipeptide class, in this work seven α-acyloxycarboxamides compounds were synthesized and subsequently the larvicidal and antimicrobial activity of these molecules was explored.
Material e métodos
The synthesis of α-acyloxycarboxamides was carried out in 3 steps, wherein the first step involved the HWE olefination reaction (3a-e) (Figure 1A), between 1 and 2 (2a-e) (3 mmol), under basic conditions. The reactional mixture was stirred over a 24 h. Subsequently, alkaline hydrolysis (Figure 1A) was performed with the cinnamic esters (3a-e) at room temperature in the presence of LiOH yielding the cinnamic acids (4a-e) (Figure 1). Then, the Passerini reaction was conducted with aromatic aldehydes (5), cinnamic acids (4a-e), and an isonitrile (6) (Figure 1A). The compounds were all characterized by ¹H, ¹³C NMR and FTIR on a Bruker instrument with a frequency of 600 MHz and a Shimadzu IR PRESTINGE equipment, respectively. The preliminary larvicidal activity to which the α-acyloxycarboxamides were subjected was carried out under the analysis of variance (ANOVA) method by employing Dunnet's method as multiple comparison analysis. The hatching of the Ae. aegypti eggs was conducted in resting tap water under temperature and humidity of 28 ± 2 ◦C and 80 ± 4%, respectively, set at 12h photoperiod. A stock solution (100µg/mL) of the α-acyloxycarboxamides was prepared with 0.11% Tween-80 (v/v). The bioassay was run out in triplicate at concentrations of stock solution of 100 µg/mL, 50 µg/mL and 5 µg/mL. The mortality of mosquito larvae was calculated after 48 h. For the positive control, temephos was employed and as a negative control, 0.11% tween-80 (v/v) was applied. The mortality rate (%M) was computed according to the WHO mortality classification (2005) and França et al. (2021). The mortality of larvae was calculated after 48 h. The percent mortality (%M) was calculated considering the mortality classification, as established by WHO, (2005) and França et al. In order to support the study, an insecticidal pharmacokinetic analysis of the compounds was conducted by using the freely available InsectiPAD web platform. Such properties have been predicted following the criteria outlined by Hao et al. (2011). The antibacterial assay for the synthetized chemical was performed in triplicate via the broth microdilution test to determine the minimum inhibitory concentration (MIC) as per CLSI guidelines. (2018). The analogues were prepared in DMSO at a stock solution of 8000µg/mL. Mueller-Hinton broth was utilized as the culture medium for S.epidermides, S. aureus and E. coli bacteria. The analogues were diluted with MHB to a concentration range between 7.8µg/mL - 1000µg/mL. The experiment was run in sterile 96-well microplates. The standard ATCC strains were used for inoculum (5 x 105 cells/mL). The positive and negative controls for this assay were Oxacillin and 0.1% DMSO (v/v), respectively. Afterwards, the assay was incubated at 35°C ± 2°C in a microbiological oven over 24 h, and MIC was set as the lowest concentration of the compound without visible growth (ZONG et al., 2019).
Resultado e discussão
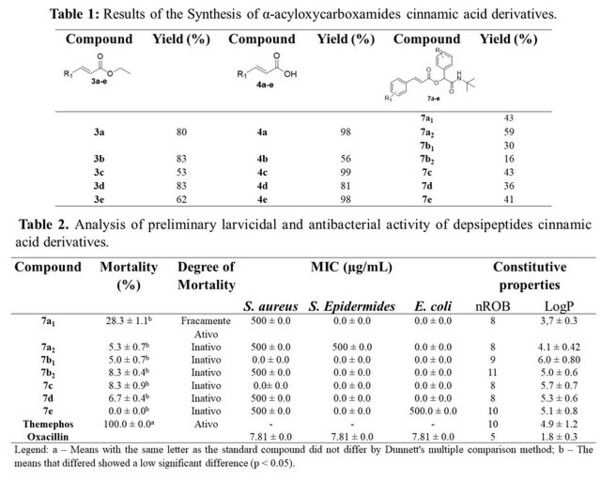
The n-substituted α-acyloxycarboxamides were synthesized by three routes, the first consisting of the Horner-Wadsworth-Emmons olefination reaction (Figure 1A) between triethyl 2-phosphonopropionate (1) and n-substituted aromatic aldehydes (2a-e), providing the n-substituted ethyl cinnamates (3a-e), with control of the geometry of the trans doublet. Compounds (3a-e) were obtained in good yields (Table 1). Soon after, an alkaline hydrolysis was carried out with the n-substituted ethyl cinnamates (3a-e) in the presence of lithium hydroxide in THF and water (2:1) at room temperature, obtaining the n-substituted cinnamic acids (4a- e) (Figure 1A) in reasonable yields (Table 1).
Figure 1. Synthesis and characterization of α-acyloxycarboxamides derivatives of cinnamic acid.
The compounds were characterized by ¹H, ¹³C NMR and Fourier transform infrared, as shown in Figure 1B. According to the spectrum, characteristic signals of 2-(tert-butylamino)-1-(4-chlorophenyl)-2-oxoethyl cinnamate (7a2) were observed, such as doublets at 6.5 ppm and 7.8 ppm, with constant coupling of J = 16.0 Hz, characteristic of the trans double bond, the signals at 7.3 to 7.6 ppm refer to the two disubstituted and monosubstituted 1,4 aromatic rings; the singlet at 6.1 ppm and 6.0 ppm (-CO-NH) belong to the asymmetric center and the amide group, respectively.
The ¹³C NMR spectrum (Figure 2C) shows the signals corresponding to compound 7a2, highlighting the carbonyl carbons of the ester and amide groups at 167.2 and 165.3 ppm, respectively; while the signals at 147.1 and 116.9 ppm refer to the olefinic carbons, the chiral carbon in turn has the chemical shift at 75.2 ppm, and the signals at 51.6 and 28.9 ppm are those corresponding to the carbons of the tert-butyl group. By the Fourier transform infrared analysis (Figure 2D), characteristic bands of this depsipeptide are also observed, among them: the stretching bands of the amide group at 3277.0 cm-1 and 3072.6 cm-1, the carbonyls of the conjugated ester and amide at 1708.9 cm-1 and 1653.0 cm-1, respectively. In addition to the double trans stretch and deformation bands at 1629.8 cm-1 and 972.1 cm-1, in that order. These results are similar to the other synthesized depsipeptides.
The depsipeptides were submitted preliminary to a larvicidal analysis, by applying three concentrations in order to evaluate the biological effect towards the fourth instar mosquito larvae. Based on Table 2, It has been found that none of the tested compounds have a pronounced larvicidal activity. Although compound 7a1 stood out in the series of depsipeptides, with a mortality rate of 28.3% (weakly active), it regressed as the concentration was reduced.
Such result may be associated with the chemical structure of the synthetized compounds, as they are large and present high hydrophobicity (Table 2). Therefore, this chemical library does not have a larvicidal action, which may be related to the amide portion, originated in the Passerini reaction, which may have inactivated the pharmacophoric fragment of cinnamic acid derivatives (ARAÚJO et al., 2021; FRANÇA et al., 2021) since França et al. (2021) have showed that the cinnamoyl moiety and the ester function are crucial for a larvicidal activity, which may be related to a possible enzymatic hydrolysis against the Ae. Aegypti mosquito larvae. In addition, studies reported by Bisel; Al-Momani & Muller. (2008) have shown that the tert-butyl group reduces the hydrolysis process due to its steric hindrance effect, inactivating the amide function, as well as the cinnamic ester.
In order to support the larvicidal bioassay, an analysis of the constitutive properties of this class of molecules was performed, as shown in Table 2. According to the insecticide similarity criteria, in addition to comparative analysis with the insecticides used in the market, the depsipeptides have high hydrophobicity and a greater number of rotating bonds, which is not in accordance with the criteria established by Rao et al. (2018). According to them, a good hydrophobicity (ClogP) and number of rotating bonds (nROB) are around ClogP ≤ 6 and nROB ≤ 9, respectively.
The compounds were also evaluated against gram positive (S. epidermides and S. aureus) and gram negative (E. coli) bacteria, proving to be inactive for both strains when compared to oxacillin (positive control) (Table 2). Although the synthesized compounds showed a pronounced effect against gram positive and negative bacteria, it was found that the α-acyloxycarboxamides 7a2 has a certain biological action against both gram-positive bacteria. These results may be associated with a greater penetration of the compounds towards the cell wall of gram-positive bacteria due to the high lipophilicity of the synthetized chemical library (Table 2). However, compound 7e also had a MIC of 500 µg/mL for E. coli despite its lack of a cell wall.
In view of the aforementioned results, a bacteriostatic and bactericidal study of the molecules that had a certain effect on the studied strains was carried out, in which it was observed that the 7a2 molecule is characterized as bacteriostatic against the S. epidermides strain, while the other molecules (7a1 – e) were bactericidal.
Therefore, although oxacillin has an antibactericidal action, it is noteworthy that the structure of α-acyloxycarboxamides may be a scaffold for the design of promising candidates with high antibacterial action since the depsipeptides contributed to the reduction or inhibition of the growth of these bacterial strains.
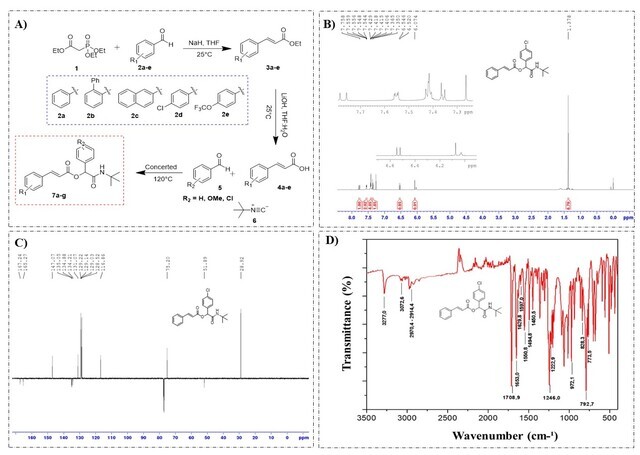
Figure 1. Synthesis and characterization of α-acyloxycarboxamides derivatives of cinnamic acid.
Conclusões
Overall, the depsipeptide derivatives were synthesized via racemic Passerini reaction. Yields were moderate with the exception of compound 7b2, for which a yield of around 16% was obtained. Therefore, the synthetic strategy for the formation of this chemical library was satisfactory since the yields obtained are in a higher range than those reported in the literature, which on average is below 40% for the only α-acyloxycarboxamides cinnamic acid derivatives existing in scientific report. Although the synthetized chemical class have not shown a larvicidal and antibactericidal profile study (which is possibly associated with the inactivation of the pharmacophoric cinnamoyl fragment - important in the larvicidal and antibacterial action - by the tert-butyl group), the α-acyloxycarboxamides can be explored for other biological targets due to its numerous activities already described in the literature.
Agradecimentos
Universidade Federal de Alagoas (UFAL); Instituto de Química e Biotecnologia, LMC, CNPq, CAPES e Fapeal.
Referências
ALVES, D. S. Estudo Investigativo do Uso de Aminoácidos Naturais na Reação de Passerini. 2014. 270 f. Dissertação (Mestrado em Química) – Universidade de Brasília, Instituto de Química, Brasília, 2014.
ARAÚJO, M. O.; PÉREZ – CASTILLO.; OLIVEIRA, L. H. G.; NUNES, F. C.; SOUZA, D. P. Larvicidal Activity of Cinnamic Acid Derivatives: Investigating Alternative Products for Aedes aegypti L. Control. Molecules, v. 26, nº 1, 10.3390, 2021.
AYOUP, M. S.; WAHBY, Y.; ABDEL – HAMID, H.; RAMADAN, M. T.; ABU – SERIE, M. M.; NOBY, A. Design, synthesis and biological evaluation of novel a-acyloxy carboxamides via Passerini reaction as caspase 3/7 activators. European Journal of Medicinal Chemistry, v. 168, 340 – 356, 2019.
BISEL, P.; AL-MOMANI, L.; M¨uller, M. The tert-butil group in chemistry and biology. Org. Biomol. Chem, v. 6, 2655 – 2665, 2008.
BOUSQUET, T.; JIDA, M.; SOUEIDAN, M.; DEPREZ – POULAIN, R.; AGBOSSOU – NIEDERCORN, F.; PELINSKI, L. Fast and efficient solvent-free Passerini reaction. Tetrahedron Letters, v. 53, 306 – 308, 2012.
FRANÇA, S. B.; DE LIMA, L. C. B.; CUNHA, C. R. S.; ANUNCIAÇÃO, D. S.; SILVA – JÚNIOR, E. F.; BARROS, M. E. S. B.; LIMA, D. J. P. Larvicidal activity and in silico studies of cinnamic acid derivatives against Aedes aegypti (Diptera: Culicidae). Bioor. Med. Chem, v. 44, 116299, 2021.
GUNIA-KRZYŻAK, A.; SŁOCZYŃSKA, K.; POPIÓŁ, J.; KOCZURKIEWICZ, P.; MARONA, H.; PĘKALA, E. Cinnamic acid derivatives in cosmetics – current use and future prospects running head: cinnamic acid derivatives in cosmetics. International Journal of Cosmetic Science, v. 40, n° 4, 356−366, 2018.
HAMADA, Y & SHIOIRI, T. Recent Progress of the Synthetic Studies of Biologically Active Marine Cyclic Peptides and Depsipeptides. Chem. Rev, v. 105, 4441 – 4482, 2005.
HAO, G.; DONG, Q.; YANG, G. A comparative Study on the Constitutive Properties of Marketed Pesticides. Mol. Inf, v. 30, 614 – 622, 2011.
IMAI, M.; YOKOE, H.; TSUBUKI, M.; TAKAHASHI, N. Growth inhibition of human breast and prostate cancer cells by cinnamic acid derivatives and their mechanism of action. Biological and Pharmaceutical Bulletin, v. 42, n°7, 1134−1139, 2019.
JIN, K.; SAM, I.H.; PO, K. H. L.; LIN, D.; ZADEH, E. H. G.; CHEN, S.; YUAN, Y.; LI, X. Total synthesis of teixobactin. Nature Communications, v. 7, 2394, 2016.
JITAREANU, A.; BALAN-PORCARASU, M.; TATARINGA, G. Cinnamic acid derivatives and 4-aminoantipyrine amides – synthesis and evaluation of biological properties. Research Journal of Chemical Sciences, v.3, n°3, 9−13, 2013.
KAZEMIZADEH, A. R & RAMAZANI, A. Passerini Multicomponent Reaction of Indane-1,2,3-Trione: an Efficient Route for the One-Pot Synthesis of Sterically Congested 2,2-Disubstituted Indane-1,3-Dione Derivatives. J. Braz. Chem. Soc, v. 20, n° 2, 309 – 312, 2009.
MARTÍNEZ-SORIANO, P. A.; MACÍAS – PÉREZ, J. R.; VELÁZQUEZ, A. M.; CAMACHO – ENRIQUEZ, B. C.; PRETELÍN – CASTILLO, G.; RUIZ – SÁNCHEZ, M. B.; ABREGO – REYES, V. H.; VILLA – TREVIÑO, S.; ANGELES, E. Solvent-free synthesis of carboxylic acids and amide analogs of CAPE (caffeic acid phenethyl ester) under infrared irradiation conditions. Green and Sustainable Chemistry, v. 5, n°2, 81−91, 2015.
PONTIKI, E.; HADJIPAVLOU-LITINA, D.; LITINAS, K.; GEROMICHALOS, G. Novel cinnamic acid derivatives as antioxidante and anticâncer agents: design, synthesis and modeling studies. Molecules, v. 19, n°7, 9655−9674, 2014.
ROGERIO, K. R.; VITÓRIO, F.; KÜMMERLE, A. E.; GRAEBIN, C. S. Reações Multicomponentes: Um breve Histórico e a Versatilidade destas Reações na Síntese de Moléculas Bioativas. Revista Virtual de Química, v. 8, n° 6, 1934 – 1962, 2016.
SAITO, M. L & LUCCHINI, F. Substancias do metabolismo secundário de plantas no controle de pragas agrícolas. LECTA, Bragança Paulista. LECTA, Bragança Paulista, v.15, n°1/2, 211−245, 1997.
SAURABH, P.; MANILA, B.; NIRAJ, T.; SONAL, P.; BANSAL, Y. K. Secondary metabolites of plants and their role: overview. Current Trends in Biotechnology and Pharmacy, v. 9, n°3, 293 – 304, 2015.
SREE, K. S.; PADMAJA, V.; MURTHY, Y. LN. Insecticidal activity of destruxin, a mycotoxin from Metarhizium anisopliae (Hypocreales), against Spodoptera litura (Lepidoptera: Noctuidae) larval stages. Pest Management Science, v. 64, 119 – 125, 2008.
WHO – Guidelines for laboratory and field testing of mosquito larvicides, World Health Organization, Geneva, 3 – 36, 2005.
ŻELECHOWSK, K.; ZAKRZEWSKI, J.; HURAS, B.; KIELCZEWSKA, A.; KRAWCZYK, M.; MICHALCZYK, A, K.; HUPKO, J.; JASZCZUK, K. Synthesis and herbicidal and fungistatic evaluation of Passerini adducts bearing phenoxyacetic moieties. Chemical Papers, v. 75, 3047 – 3059, 2021.
ZONG, Y.; FANG, F.; MEYER, K. K. J.; WANG, L.; NI, Z.; GAO, H.; LEWIS, K.; ZHANG, J.; RAO, Y. Gram-scale total synthesis of teixobactin promoting binding mode study and discovery of more potent antibiotics. Nature Communications, v. 10, 3268, 2019.
















