Autores
Ardila, D. (UNIVERSIDAD INDUSTRIAL DE SANTANDER) ; Vera, D. (UNIVERSIDAD INDUSTRIAL DE SANTANDER) ; Rodríguez, D. (UNIVERSIDAD INDUSTRIAL DE SANTANDER) ; álvarez, G. (UNIVERSIDAD INDUSTRIAL DE SANTANDER) ; Palma, A. (UNIVERSIDAD INDUSTRIAL DE SANTANDER) ; Cobo, J. (UNIVERSIDAD DE JAÉN)
Resumo
A simple and effective two-step approach to afford a novel series of chromone-
quinoline molecular hybrids is reported. Starting from 2-methyl-4-
styrylquinolines, reaction with selenium dioxide yields the corresponding 2-
formyl-4-styrylquinolines, which then react with 2´-hydroxyacetophenone to give
the target molecular hybrids of the type (E)-2-(4-styrylquinolin-2-yl)-4H-chromen-
4-ones in good yields. Molecular structures of the obtained molecular hybrids were
fully determined by IR, HRMS and NMR spectroscopy.
Palavras chaves
styrylquinolines; chromones; molecular hybridization
Introdução
Molecular hybridization constitutes a useful rational strategy for the synthesis
of new chemical libraries. This strategy is based on the combination of two or
more pharmacophoric moieties of different bioactive compounds to produce a new
molecular hybrid with more favorable biological activity (Claudio Viegas-Junior
et al, p 1829, 2007. Different privileged structures have been used for the
design and discovery of novel molecular hybrids being quinoline scaffold one of
the most commonly employed due to their wide occurrence in natural and synthetic
biologically active compounds, and by the large number of quinoline therapeutic
agents that have been developed and are currently prescribed in clinical
treatments.(Matada, et al, p 115973, 2021; Orozco, et al, p 4876, 2020).
Amongst the different quinoline derivatives, styrylquinolines are of continued
interest to organic and medicinal chemistry as they have been recognized as
promising anticancer, anti-HIV, anti-Alzheimer, antiparasitic, antiviral, and
antibacterial agents (El-Sayed et al, p 199, 2018). Chromone ring is another
privileged scaffold that is broadly present in the structures of myriad natural
and synthetic bioactive compounds and drugs (Keri et al, p 340 2014; Mohsin et
al, p 241, 2020). Guided by the molecular hybridization concept, many authors
have reported the synthesis of molecular hybrids formed by the combination of
quinoline and chromone moieties with structurally diverse chemicals with
amplified or new bioactivities (Kumar et al, p 1895, 2019; Mungra et al, p 4192,
2011). Encouraged by the above-mentioned considerations, here, we described an
alternative and efficient two-step procedure for the synthesis of novel
chromone-quinoline hybrids 3 using well-known classical reactions
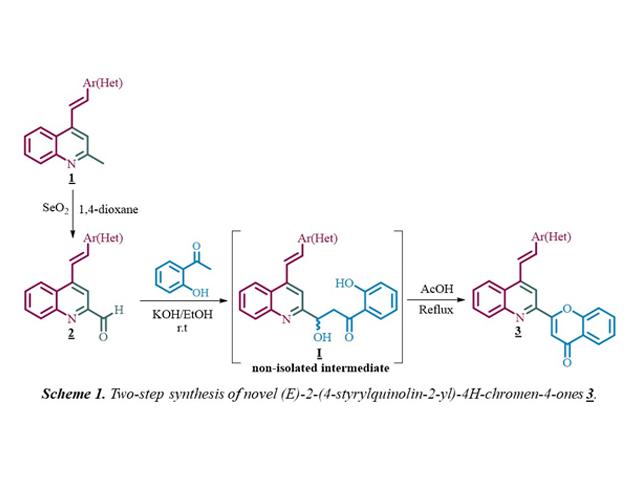
Material e métodos
The (E)-2-methyl-4-styrylquinolines 1, previously prepared by the
Friedländer reaction between 2´-aminophenylchalcones and excess of acetone in
glacial acetic acid, were transformed into the intermediate (E)-4-
styrylquinoline-2-carbaldehydes 2, by treatment with selenium
dioxide (2 equiv.) in 3 mL of 1,4-dioxane at 100 °C through an oxidative
reaction. Then, the synthesis of the target chromone-quinoline molecular hybrids
3 was carried out in a two-step one-pot process that involves, in
the first step, a crossed aldol condensation reaction between 2-
formylderivatives 2 (1.0 mmol) and 2´-hydroxyacetophenone (1.0
mmol) in ethanolic solution of KOH (2 % w/v) at room temperature to produce the
non-isolated intermediate (E)-2-(2-hydroxy-2-(4-styrylquinolin-2-
yl)ethyl)phenols I, which in the second step, after the pH of the
medium was set at 4 by adding acetic acid (2.3 mL/mmol of KOH) and the
temperature was increased to 110 °C, undergo both the dehydration and the
concomitant oxidative cyclization to yield the expected novel hybrids
3.
Resultado e discussão
As depicted in Scheme 1, the methyl group at C-2 position of the 2-methyl-4-
styrylquinolines 1 was selective and effectively oxidized using
selenium dioxide as oxidizing agent to obtain the corresponding 2-formyl
derivatives 2 in short reaction times (1-2 hours) as colorless
solids with high yields (>90%). With the key precursors 2 in
hands, we next turned our attention to the transformation of 2
into the desired hybrids 3 by reaction with 2´-hydroxyacetophenone
under classical Claisen-Schmidt condensation conditions at room temperature. In
a first experiment, after 6-8 hours, instead the expected chalcone only the
formation of the aldol-intermediate I was observed. This
intermediate was isolated and characterized by NMR spectroscopy. Further
attempts to promote the dehydration of I by reaction with acetic
acid at 110 °C resulted in the formation of the corresponding hybrid 3
. Taking into account the above results, and with the aim to achieve a more
efficient synthesis of 3, we decided not to isolate intermediate
I but add the appropriate amount of AcOH to set pH = 4 and
increase the temperature from room temperature to 110 °C. Fortunately, under
such reaction conditions all the formyl derivatives 2 were
transformed exclusively, through aldol-intermediate formation, dehydration, and
concomitant oxidative cyclization of aldol intermediate, into the corresponding
molecular hybrids 3, which were obtained in good yields (61-75%)
and as pale-yellow solids with well-defined melting points. Finally, structures
of the novel chromone-quinoline 3 were confirmed by IR, NMR and
HRMS.
Conclusões
Twelve novel chromone-quinoline hybrids 3 were successfully synthesized in good
yields using an novel two-step methodology developed, which consists in a
selective oxidation of 2-metyl-4-styrylquinolines following by a one-pot two-step
procedure that involves crossed aldol condensation between formyl derivatives 2
and 2-hydroxyacetophenone and subsequent oxidative cyclization reaction of the
non-isolated aldol intermediate. The present approach offers the advantages to
access to novel quinoline hybrids that incorporate in their structure a styryl
fragment at C-4 and a chromone nucleus at C-2.
Agradecimentos
Authors acknowledge for the financial support to the Vicerrectoría de
Investigación y Extensión of the Universidad Industrial de Santander (Grant No
2680).
Referências
Claudio Viegas-Junior, Eliezer J. Barreiro, and Carlos Alberto Manssour Fraga. 2007. “Molecular Hybridization: A Useful Tool in the Design of New Drug Prototypes.” Current Medicinal Chemistry 14(17): 1829–52.
El-Sayed, Magda A.A. et al. 2018. “Synthesis and Biological Evaluation of 2-Styrylquinolines as Antitumour Agents and EGFR Kinase Inhibitors: Molecular Docking Study.” Journal of Enzyme Inhibition and Medicinal Chemistry 33(1): 199–209.
Keri, Rangappa S., Srinivasa Budagumpi, Ranjith Krishna Pai, and R. Geetha Balakrishna. 2014. “Chromones as a Privileged Scaffold in Drug Discovery: A Review.” European Journal of Medicinal Chemistry 78: 340–74.
Kumar, T. Uday, Durba Roy, and Anupam Bhattacharya. 2019. “Iron(III) Catalyzed Direct C–H Functionalization at the C-3 Position of Chromone for the Synthesis of Fused Chromeno-Quinoline Scaffolds.” Tetrahedron Letters 60(29): 1895–98.
Matada, Basavarajaiah Suliphuldevara, Raviraj Pattanashettar, and Nagesh Gunavanthrao Yernale. 2021. “A Comprehensive Review on the Biological Interest of Quinoline and Its Derivatives.” Bioorganic and Medicinal Chemistry 32(December 2020): 115973.
Mohsin, Noor ul Amin, Muhammad Irfan, Shams ul Hassan, and Usman Saleem. 2020. “Current Strategies in Development of New Chromone Derivatives with Diversified Pharmacological Activities: A Review.” Pharmaceutical Chemistry Journal 54(3): 241–57.
Mungra, Divyesh C., Manish P. Patel, Dhanji P. Rajani, and Ranjan G. Patel. 2011. “Synthesis and Identification of β-Aryloxyquinolines and Their Pyrano[3,2-c]Chromene Derivatives as a New Class of Antimicrobial and Antituberculosis Agents.” European Journal of Medicinal Chemistry 46(9): 4192–4200.
Orozco, Dayana et al. 2020. “Recent Synthetic Efforts in the Preparation of 2-(3,4)-Alkenyl (Aryl) Quinoline Molecules towards Anti-Kinetoplastid Agents.” RSC Advances 10(9): 4876–98.
















