Autores
Vera, D. (UNIVESIDAD INDUSTRIAL DE SANTANDER) ; Mantilla, J. (UNIVESIDAD INDUSTRIAL DE SANTANDER) ; Ardila, D. (UNIVESIDAD INDUSTRIAL DE SANTANDER) ; Rodríguez, D. (UNIVESIDAD INDUSTRIAL DE SANTANDER) ; Palma, A. (UNIVESIDAD INDUSTRIAL DE SANTANDER) ; Cobo, J. (UNIVERSIDAD DE JAÉN)
Resumo
An alternative and efficient three-step approach to access novel styrylquinoline-
chalcone molecular hybrids 4 is reported. The easily accessible 2’-
aminophenylchalcones 1 were used as starting materials to
synthetize, via the Friedländer reaction, the required key intermediates 2-methyl-
4-styrylquinolines 2. Oxidation of the latter afforded the
corresponding formyl derivatives 3 which, in turn, via the Claisen-
Schmidt condensation, were transformed into the target styrylquinoline-chalcones
by reaction with 1(2)-naphthaldehydes.
Palavras chaves
Quinoline-Chalcone hybrid; Styrylquinoline; Claisen-Schmidt reaction
Introdução
Styrylquinolines represent an outstanding class of quinoline derivatives that
have been recently investigated by the organic and medicinal chemistry, as a
consequence of the broad spectrum of biological activity they have exhibited.
(MUSIOL, p. 141, 2020) Chalcones are also highly privileged structures in drug
discovery. Such fragments are found very frequently in biologically active
compounds and thus are common building blocks for drugs and natural product
derivatives.(GOMEZ et al., p. 1210, 2017; NASIR et al., p. 1394, 2012) the other
hand, quinoline-chalcone molecular hybrids have found to manifest an extensive
number of biological properties being the antifungal, antibacterial,
antimalarial, analgesic, anti-VIH and anticancer activities, the most
representatives.(ATUKURI et al., p. 104419, 2020; CHAYA et al., p. 338, 2022;
UGWU et al., p. 459, 2015; VERMA et al., p. 22, 2018) In the course of our
research toward novel heterocyclic scaffolds, (MELÉNDEZ et al., p. 1804, 2020)
our efforts were directed towards the synthesis of unreported styrylquinoline-
chalcone molecular hybrids of the type 4. Starting from 2’-
aminophenylchalcones 1, derived from 2’-aminoacetophenone and
different aromatic aldehydes, the aim was to prepare the key precursor 2-methyl-
4-styrylquinolines 2 for further functionalization under the
selective oxidation-Claisen-Schmidt condensation sequence.
Material e métodos
The 2-methyl-4-styrylquinolines 2 were synthetized through the
Friedländer reaction between synthetically available 2’-aminophenylchalcones
1 (1.0 mmol) and excess of acetone (12.0 mmol) in glacial acetic acid
(3.0 mL/1.0 mmol of 1) at 80 °C. Then, the precursors 2
(1 mmol) were selectively oxidized in 1,4-dioxane at 100 °C utilizing
SeO2 (2 equiv.) as an oxidizing agent, to obtain the corresponding 2-
formilquinoline derivatives 3. Finally, formil derivatives
3 were transformed into the target quinoline-chalcone molecular hybrids
4 by the Claisen-Schmidt condensation with 1-(naphthalen-1(2)-
yl)ethan-1-ones in ethanolic potassium hydroxide solution at room temperature.
Resultado e discussão
As shown in Scheme 1, the Friedlander reaction between 2’-aminophenylchalcones
1 and acetone was effectively carried out, delivering the
expected key precursor 2 as the sole product in yields of 71-93%.
The intermediate 4-styryl-2-methylquinolines 2 then, underwent
oxidation to afford, the formyl derivatives 3 in excellent yields
(> 89%). As was evidenced from NMR data, both the Friedländer products
2 and the oxidized products 3 did not suffer any
modification in the E stereochemistry of the styryl fragment. Finally,
the desired molecular hybrids 4 were obtained in high yields (>
81%), after 1-8 hours of reaction, by reacting 2-formylquinolines 3
with 1-(naphthalen-1(2)-yl)ethan-1-ones under the well-known Claisen-
Schmidt condensation conditions. The novel styrylquinoline-chalcone hybrids
4 were isolated as solids with well-defined melting points and fully
characterized by the commonly used spectroscopy techniques such as IR, NMR, HR-
MS and XRD. The formation of molecular hybrids 4 was established
by disappearance of the formyl hydrogen signal (δ10.23-10.26 and 193.9-194.2) in
both the 1H and 13C NMR spectra and by the appearance of
signals for the newly formed chalcone (arylpropenone) fragment. The Claisen-
Schmidt condensation proceeded in a highly stereoselective manner giving
exclusively the E stereoisomer, configuration deduced based on coupling
constantan values (3JHA’/HB’ ≈16.0
Hz) between HA’ and HB’, whose signals in the
1H NMR spectra appear at δ7.91-8.41 and 7.78-8.07, respectively.
Furthermore, single crystal X-ray diffraction of 4a, 4b
and 4j confirmed the stereochemical assignment for this class
of molecular hybrids.
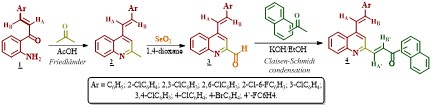
[b][u] Scheme 1. [/u][/b] Synthetic sequence to afford the novel quinoline-chalcone molecular hybrids [b][u]4[/u][/b].
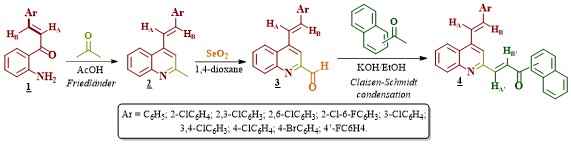
[b][u] Scheme 1. [/u][/b] Synthetic sequence to afford the novel quinoline-chalcone molecular hybrids [b][u]4[/u][/b].
Conclusões
We have developed a reliable three-step synthetic approach with advantages such as
readily available starting materials, mild reaction conditions and high yields for
the assembly of novel quinoline-based hybrid molecules containing both styryl and
chalcone moieties. Most of the synthetized molecular hybrids 4 were
selected by the National Cancer Institute, USA, for investigation of antitumor
activity under their drug discovery program. This study is in progress and will be
the subject of another report.
Agradecimentos
Authors gratefully acknowledge the financial support for this research from the
Vicerrectoría de Investigación y Extensión of the Universidad Industrial de
Santander (Project 2680).
Referências
ATUKURI, D., VIJAYALAXMI, S., SANJEEVAMURTHY, R., VIDYA, L., PRASANNAKUMAR, R. & RAGHAVENDRA, M. Identification of quinoline-chalcones and heterocyclic chalcone-appended quinolines as broad-spectrum pharmacological agents. Bioorganic Chemistry, 105, 104419–104450. (2020).
CHAYA, P., CHERIYAN, A. A., SHAH, S., KRISHNAN, A. & THOMAS, L. Synthesis and medicinal applications of quinoline hybrid heterocycles : a comprehensive review Molecular Chemistry. Journal of Molecular Chemistry, 22(1), 338–379. (2022).
GOMES, M. N., MURATOV, E. N., PEREIRA, M., PEIXOTO, J. C., ROSSETO, L. P., CRAVO, P. V. L., ANDRADE, C. H., & NEVES, B. J. Chalcone derivatives: Promising starting points for drug design. Molecules, 22(8), 1210–1234. (2017).
MELÉNDEZ, A., PLATA, E., RODRIGUEZ IBAÑEZ, D., ARDILA, D., GUERRERO, S., ACOSTA, L., COBO, J., NOGUERAS, M., & PALMA, A. Straightforward Synthesis of Novel 4-Styrylquinolines/4-Styrylquinolin-2-ones and 9-Styryldihydroacridin-1(2H)-ones from Substituted 2′-Aminochalcones. Synthesis, 52(12), 1804–1822. (2020).
MUSIOL, R. Styrylquinoline – a versatile scaffold in medicinal chemistry. Medicinal Chemistry, 16(2), 141–154. (2020).
NASIR, S., JASAMAI, M., & JANTAN, I. Synthesis and Biological Evaluation of Chalcone Derivatives (Mini Review). Mini-Reviews in Medicinal Chemistry, 12(13), 1394–1403. (2012).
UGWU, D. I., EZEMA, B. E., OKORO, U. C., EZE, F. U., EKOH, O. C., EGBUJOR, M. C., & UGWUJA, D. I. Synthesis and Pharmacological Applications of Chalcones- a Review. Int. J. Chem. Sci, 13(1), 459–500. (2015).
VERMA, S., SRIVASTAVA, A. K., & PANDEY, O. P. A Review on Chalcones Synthesis and their Biological Activity. Pharmatutor, 6(2), 22–39. (2018).
















