Autores
dos Santos Filho, J.M. (UFPE) ; de Souza Castro, M.V.B. (UFPE)
Resumo
The search for new biological active and structurally diverse molecules is the
core of medicinal chemistry, which can be achieved by different theoretical and
experimental strategies, including molecular simplification. Starting from a more
complex structural model, it is possible to design new molecules with easier
synthetical requirements, improved physicochemical properties, and even new
biological profiles. Based on this strategy, 10 ferrocenyl-N-acyl hydrazone
(Fc-NAH) hybrids have been planned, synthesized, and screened for their
antimicrobial activity. In opposition to the starting structural model, the Fc-NAH
derivatives have disclosed significant antimicrobial activity, especially against
the gram-positive bacterial strains B. subtilis and S. aureus.
Palavras chaves
molecular simplification; Fc-NAH; antimicrobial activity
Introdução
The association of the ferrocene (Fc) scaffold with recognized pharmacophoric
structures has led to important improvements in the biological activities of
resulting molecules, confirming its importance for medicinal chemistry [SINGH
et al, 2019]. Ferrocene-bearing derivatives are associated with a
plethora of biological activities [LARIK et al, 2016], among them the
antimicrobial activity [HASSAN and HAFEZ, 2018]. Additionally, the N-acyl
hydrazone (NAH) moiety is, just like ferrocene, associated with several
biological activities, and has been recognized as a privileged structure in
medicinal chemistry [VERMA et al, 2014]. Analyzing the structure of
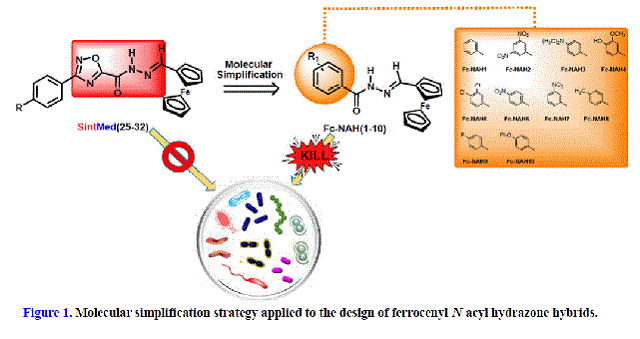
derivatives SintMed(25-32), another usual strategy applied in medicinal
chemistry, the molecular simplification [WANG et al, 2019], seems to be
a natural and promising approach for the discovery of new bioactive molecules.
Compounds SintMed(25-32) were screened for their antimicrobial profile and found
to be inactive. The removal of the 1,2,4-oxadiazole ring leads to smaller
molecules, whose preparation could be easier, faster, and less expensive could
disclose a larger diversity of structures, potentializing the discovery of
biologically active products, especially considering that the simplified
structures still retain the important N-acyl hydrazone moiety (Figure 1).
Indeed, 10 novel ferrocene-N-acyl hydrazones Fc-NAH(1-10) were prepared,
characterized, and screened for their antimicrobial activity. Gratifyingly, the
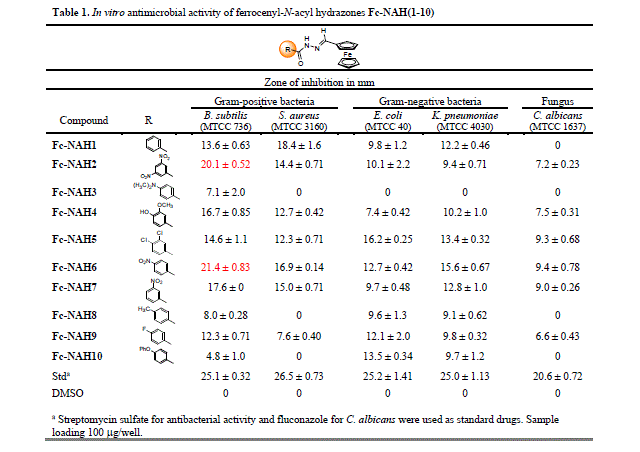
Fc-NAH compounds have displayed antimicrobial activity for all tested strains of
microorganisms, as observed in Table 1. The best outcomes were found for the
gram-positive bacteria B. subtilis and S. aureus, evidencing the
success of the structural simplification strategy in the discovery of new
biologically active compounds. The susceptibility tests were performed following
the agar well diffusion method for the antimicrobial and antifungal activities,
according to the established guidelines [SWETHA et al, 2019].
Material e métodos
Compounds Fc-NAH(1-10) were previously prepared and characterized by
spectroscopic methods before undergoing the biological investigation. The
antimicrobial activity assays were performed using the agar well diffusion
method for both antibacterial and antifungal activities. All the synthesized
compounds were evaluated for their in vitro antimicrobial activity
against two Gram-positive bacteria, viz., Bacillus subtilis (MTCC 736)
and Staphylococcus aureus (MTCC 3160), and two Gram-negative bacteria,
viz., Escherichia coli (MTCC 40) and Klebsiella pneumoniae (MTCC
4030) along with one fungal strain Candida albicans (MTCC 1637), which
were provided from the culture collection of the Microbiology Laboratory of the
Universidade Federal de Pernambuco. Nutrient agar (1.3%) was used for the growth
of bacteria, and YPD (yeast extract 1%, peptone, and dextrose 2%) agar for
fungus. Streptomycin sulfate and fluconazole were used as standard drugs for
antibacterial and antifungal activity evaluation, respectively. All the
synthesized compounds and standards were diluted with DMSO to get a final
concentration of 1 mg/mL. The medium and the Petri plates were sterilized under
standard conditions. After sterilization, the medium was poured into Petri
plates and allowed to solidify. After solidification, 60 mL of the test organism
was inoculated and uniformly spread over the agar surface with a sterile L-
shaped rod. Wells (8 mm) were made with a sterile cork borer and exactly 100 μL
of the respective test samples and standard were placed in separate wells on
each plate. The plates were first incubated for 20-30 min at 4°C to allow the
compounds to diffuse into the agar, and then subsequently incubated for 24 h at
37°C for antibacterial and 48 h for antifungal activity. Inhibition zones were
measured and the diameter was calculated in millimeters (mm) using a calibrated
scale. The experiments were performed in triplicate and average values were
reported to minimize the deviations [SWETHA et al, 2019]. All results
were reported in Table 1.
Resultado e discussão
The susceptibility tests were performed following the agar well diffusion method
for the antimicrobial and antifungal activities at the concentration of 100
μg/well, according to the established guidelines. The derivatives Fc-NAH(1-10)
were evaluated for their in vitro antimicrobial activity against two gram-
positive
bacteria, along with one fungal strain. Results were given in terms of zone of
inhibition in millimeters (mm). Besides, the ferrocene-N-acyl hydrazone-
1,2,4-oxadiazole derivatives SintMed(25-32) also have undergone antimicrobial
screening, in order to establish their biological profile, and appraise how the
molecular simplification strategy has affected the biological response of
compounds Fc-NAH(1-10). Results for the series SintMed(25-32) have unequivocally
shown their inactivity against all microorganisms under experimental conditions
(Figure 1).
The Fc-NAH compounds have displayed antimicrobial activity for all strains of
microorganisms, as observed in Table 1. The best outcomes were found for the
gram-positive bacteria, especially for the B. subtilis. The first and
simplest example of the series is the derivative Fc-NAH1, bearing an
unsubstituted phenyl ring, which has exhibited the best activities against B.
subtilis and S. aureus, both relevant results when compared to the
standard antimicrobial drug. Besides, this molecule has presented a good
inhibition halo for E. coli and K. pneumoniae, in opposition to
its complete inactivity against C. albicans. In comparison, substituted
compounds Fc-NAH2 (3,5-dinitrophenyl) and Fc-NAH6 (4-nitrophenyl) have shown
remarkable inhibition zones against B. subtilis, close to the standard
drug streptomycin sulfate, and have in common the presence of nitro substituents
in their structures. This structural feature is also observed for derivative
Fc-NAH7 (3-nitrophenyl), whose activity was a little lower than the other
nitro-bearing compounds. The three nitro-substituted derivatives haven’t
retained their biological profile against the S. aureus strain, although
their antimicrobial activity was yet good. These three nitro-substituted
derivatives have performed moderately against gram-negative bacteria E.
coli and K. pneumoniae. Observing the overall results for the fungus
C. albicans, the three derivatives Fc-NAH2(3,5-dinitrophenyl), Fc-NAH6
(4-nitrophenyl), and Fc-NAH7 (3-nitrophenyl) have exhibited some activity,
indicating that the nitro substitution can be closely related to the
antimicrobial response.
Since the nitro group is a strong electron-withdrawing substituent, this
specific characteristic was considered an important factor for the general
antimicrobial response. However, compound Fc-NAH9 (4-fluorophenyl) has disclosed
moderate responses for B. subtilis and E. coli. These results are
similar for the derivatives Fc-NAH4 (3-methoxy-4-hydroxyphenyl) and Fc-NAH5
(3,4-dichlorophenyl), whose antibacterial activities were moderate, while the
antifungal activity was low.
The worse antimicrobial outcomes were observed for compounds Fc-NAH3 (4-
dimetylaminophenyl), Fc-NAH8 (4-tolyl), and Fc-NAH10 (4-phenoxyphenyl). These
three compounds bear lipophilic substituents, suggesting that this specific
structural feature affects negatively the biological response. The derivative
Fc-NAH3 has displayed the poorest results, being inactive for almost all
microorganisms, except for B. subtilis.
Conclusões
Herein a successful strategy of structural simplification was applied in the
design of a series of novel ferrocenyl-N-acyl hydrazones (Fc-NAH), starting from a
more complex structural leitmotiv. The preliminary biological investigation using
the agar well diffusion method has brought in evidence that all synthesized Fc-
NAH(1-10) have presented antimicrobial activity, especially against the gram-
positive bacterial strain B. subtilis, as well as against S. aureus. Nitro-
substituted derivatives, namely, Fc-NAH2 (3,5-dinitrophenyl), Fc-NAH6 (4-
nitrophenyl), and Fc-NAH7 (3-nitrophenyl) have exhibited the best inhibition
values, establishing the structural features for the development of new
antimicrobial agents. The outcomes have brought to light the evidence that
lipophilic substitution leads to the loss of antimicrobial activity, establishing
a relevant structure-activity relationship (SAR) for the series Fc-NAH, which can
be useful for the development of further studies.
Agradecimentos
Special acknowledgment to the Laboratory of Microbiology of the Universidade
Federal de Pernambuco for the technical support in the management of the
microorganism culture library.
Referências
HASSAN, A.S., HAFEZ, T.S., Antimicrobial Activities of Ferrocenyl Complexes: A Review, J App Pharm Sci. 8 (5) (2018), 156-165.
LARIK, F.A., SAEED A., FATTAH, T.A., MUQADAR U., CHANNAR, P.A. Recent advances in the synthesis, biological activities and various applications of ferrocene derivatives, Appl. Organometal. Chem. (2016) 1-22.
SINGH, A., LUMB, I., MEHRA, V., KUMAR, V. Ferrocene-appended pharmacophores: an exciting approach for modulating the biological potential of organic scaffolds, Dalton Trans. 48 (2019) 2840-2860.
SWETHA, Y., REDDY, E.R., KUMAR, J.R., TRIVEDI, R., GIRIBAU, L., SRIDHAR, B., RATHOD, B., PRAKASHAM, R.S. Synthesis, characterization and antimicrobial evaluation of ferrocene–oxime ether benzyl 1H-1,2,3-triazole hybrids, New J. Chem. 43 (2019) 8341-8351.
VERMA, G., MARELLA, A., SHAQUIQUZZAMAN, M., AKHTAR, M., ALI, M.R., ALAM, M.M., A review exploring biological activities of hydrazones, J. Pharm. Bioallied. Sci. 6 (2) (2014), 69-80.
WANG, S., DONG, G., SHENG, C., Structural simplification: an efficient strategy in lead optimization, Acta Pharm. Sin. B, 9 (5) (2019) 880-901.
















