Autores
Ortega de Oliveira, P.C. (UNIVERSIDADE FEDERAL FLUMINENSE) ; Rodrigues Coelho Medeiros, P. (UNIVERSIDADE FEDERAL FLUMINENSE) ; Albino, M. (UNIVERSITÀ DEGLI STUDI DI FIRENZE) ; Muzzi, B. (UNIVERSITÀ DEGLI STUDI DI FIRENZE) ; Sangregorio, C. (UNIVERSITÀ DEGLI STUDI DI FIRENZE) ; Wanderley Tinoco, L. (UNIVERSIDADE FEDERAL DO RIO DE JANEIRO) ; Alves Romeiro, G. (UNIVERSIDADE FEDERAL FLUMINENSE) ; de Moraes, M.C. (UNIVERSIDADE FEDERAL FLUMINENSE)
Resumo
LdNH is an important biological target to screen potential leishmanicidal
compounds. In order to optimize an LdNH inhibitor screening methodology and
contribute to the drug discovery process, this work evaluates the application of
NP-SS (M = 49 Am2.Kg-1) and NP-LE (M = 76
Am2.Kg-1) magnetic nanoparticles in the immobilization of
LdNH. The two LdNH-NPs systems presented KM values of
797.3 ± 71.7 µmol.L-1 and 486.5 ± 30.0 µmol.L-1, and
immobilization yields of 67% and 53%, respectively. The higher magnetization value
and lower KM show that NP-LE is promising solid support and it can be
applied to screen new inhibitors
Palavras chaves
Screening assay; Magnetic support; Nucleoside Hydrolase
Introdução
The protozoa of Leishmania donovani (Ld) specie is the causative
agent of the most brutal form of leishmaniasis, visceral leishmaniasis, related
to a high rate of death in untreated cases. The available treatment uses highly
toxic and expensive drugs, with reports of treatment resistance (FIGUEROA-
VILLAR; SALES, 2017; FRANÇA et al, 2008). The scenario raises awareness of the
necessity of alternative drugs. The enzyme Nucleoside Hydrolase (NH) acts on the
Ld purine salvage pathway, hydrolyzing the host’s ribonucleoside to ribose and
purine that will compose the RNA and DNA of the protozoan. The NH role is
essential for the parasite’s survival and therefore is considered a promising
biological target for the development of new leishmanicidal agents. Immobilizing
a biological target to solid support brings many advantages, such as stability
to temperature and pH changes. Among the many available options, magnetic
particles (MPs) have a prominent place due to their characteristics: easy and
fast recovery when applying an external magnetic field; simple surface
functionalization; and high surface area. The applications of MP in the
biological area are extensive, this support can be applied in hyperthermia
studies, drug delivery, screening assays, and more (TRINDADE XIMENES et al,
2021). Regarding screening assay the use of MP is paramount, this support,
allows a series of system configurations highlighting its versatility. For that
matter, developing solid supports with interesting characteristics has a lot to
contribute to the performance of screening assays. This work focus on using
magnetic nanoparticles as support for the immobilization of Nucleoside Hydrolase
from Leishmania donovani (LdNH) in the development of a screening
assay.
Material e métodos
The iron oxide nanoparticles (NP) were obtained by the thermal decomposition of
iron (III) acetylacetonate in benzyl ether, using oleic acid as a stabilizing
agent. After characterization (to define crystalline phase, particle diameter
size, and magnetic response), the amino group was introduced onto the particle’s
surface using two approaches: a) through a functionalized silica shell (SS), and
b) by changing the stabilizing agent (LE). The -NH2 groups allowed
the LdNH immobilization through a crosslinking agent, glutaraldehyde,
forming a Schiff base. The immobilization performance was measured using the
immobilization’s yield, through the quantification of enzyme in solution prior
to and after the immobilization process using Lowry’s methodology to quantify
proteins; and the value obtained for the Michaelis Menten constant
(KM). The reuse response of the LdNH-NP was also evaluated
through a series of cycles, in which the catalytic activity was determined after
each cycle. The enzyme activity was monitored by HPLC-DAD where the substrate
(inosine) and product (hypoxanthine) were chromatographically separated by an
octadecyl column (Supelco Ascentis Express C18 5 µm 5.0 x 0.46 cm) with a mobile
phase composed by a solution containing triethylamine (1% in water, v/v,
acidified with AcOH pH 6.0): MeOH (95:5, v/v) at flow rate 0.8
mL.min-1.
Resultado e discussão
The characterization of the nanoparticle showed that it was obtained a 25 nm
magnetite particle with the magnetization of 85 Am2.Kg-1.
After functionalization, the magnetization of NP presented 49
Am2.Kg-1 for the NP with silica shell (NP-SS) and 76
Am2.Kg-1 for NP with ligand exchange (NP-LE). The
difference in magnetization values is expected considering the distinguished
approach to coat the NP surface. The yield of immobilization (YI) and the
kinetic studies (KM) for obtained for NP-SS (YI = 67%; KM
= 797.3 ± 71.7 µmol.L-1) and NP-LE (YI = 53%; KM = 486.5 ±
30.0 µmol.L-1) showed that the LdNH immobilization achieved
satisfactory results. Nonetheless, the kinetic study revealed a distinct
behavior, the NP-LE presented a lower value for KM this indicates
that the enzyme in this system has more affinity for the substrate than in the
other particle. The KM obtained for NP-LE was, relatively, close to
the KM obtained for the free enzyme in solution KM = 370
µmol.L-1 (ALVES et al, 2016). In the next step, the reusability of
the NPMs coated with LdNH was evaluated in 5 consecutive reaction cycles
(Figure 1). In general, the NPs showed similar behavior in the study. After the
second cycle, there is a tendency to stabilize. This indicates that both systems
can be reused for several cycles, which is an important parameter for screening
assays.
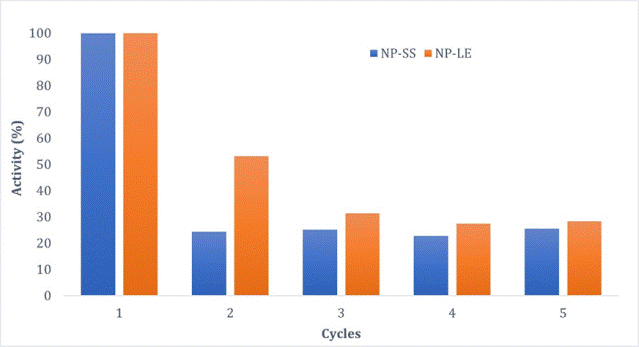
Reuse study of nanoparticles
Conclusões
The ligand exchange procedure resulted in an NP with a surface more suitable to
immobilize the LdNH enzyme illustrated by the KM, regardless of its lower
immobilization yield. The reusability study showed that both samples behaved
similarly. NP-LE was able to retain more activity during the cycles and the reason
for that relies on this magnetization value. These particles have a stronger
response to the external magnetic field. NP-LE is a good candidate to develop
screening assays to compose an immobilized enzyme reactor that used an inline
methodology to screen LdNH inhibitors.
Agradecimentos
CNPq, FAPERJ, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil
(CAPES) – Finance Code 001 e CAPES PrInt.
Referências
ALVES, M. A. et al. Non-competitive inhibitor of nucleoside hydrolase from Leishmania donovani identified by fragment-based drug discovery. RSC Advances, v. 6, n. 90, p. 87738–87744, 2016.
FIGUEROA-VILLAR, J. D.; SALES, E. M. The importance of nucleoside hydrolase enzyme (NH) in studies to treatment of Leishmania: A review. Chemico-Biological Interactions, v. 263, p. 18–27, 2017.
FRANÇA, T. C. C. et al. Design of Inhibitors for Nucleoside Hydrolase from Leishmania donovani using Molecular Dynamics Studies. Journal of the Brazilian Chemical Society, v. 19, n. 1, p. 64–73, 2008.
TRINDADE XIMENES, I. A. et al. Magnetic particles for enzyme immobilization: A versatile support for ligand screening. Journal of Pharmaceutical and Biomedical Analysis, v. 204, p. 114286, 2021.
















