Autores
Naziozene, G.M.O.S. (UNIVERSIDADE DE SÃO PAULO) ; Meira, P.A. (UNIVERSIDADE DE SÃO PAULO) ; Palma, M.S.A. (UNIVERSIDADE DE SÃO PAULO)
Resumo
The present study aimed at the synthesis of Dropropizine. It was synthesized
through the reaction of Phenylpiperazine with Glycidol, aiming the transposition
from the batch process to flow in microreactor.The maximum yield, 92.8%, was
obtained for the flow reaction at 80 °C, 12 min mean residence time and 0.8 M
total concentration. For the reactions at 100 °C, the reaction medium showed a
color change, from yellowish to orange, and reaction yield higher than 100%,
suggesting, possibly, parallel/consecutive reactions yielding by-products that
could absorb UV for the same wavelength and same retention time of Dropropizine.
Continuing this study, experiments will be replicated at 100 °C to confirm the
results and it will be tried to identify the by-product formed at that high
temperature.
Palavras chaves
Dropropizine; Microreactor; Flow Chemistry
Introdução
The reduction of effluent generation and the search for higher efficiency in the
use of energy and material resources are crucial factors for a sustainable
technical-scientific development (JIMÉNEZ-GONZÁLEZ; CONSTABLE; PONDER, 2012). If
the materials and energy required for the synthesis of substances are reduced,
consequently, the production processes will be less harmful to environment and
more sustainable.The flow synthesis using capillary microreactors has been the
object of study in recent years due to its wide advantages over batch reactors
(SILVA et al.,2019) that lead to increased chemical reaction rate, conversion,
yield, selectivity and safety when working with toxic reagents and products,
thus reducing waste generation (YOSHIDA; TAKAHASHI; NAGAKI, 2013). Dropropizine
is an antitussive that acts by inhibiting the cough reflex through its action on
peripheral receptors and their afferent conductors (MACHADO et al,2021). Its
chemical name IUPAC is 3-(4-phenyl-1-piperazinil)-1,2-propanediol and has an
optical center. The objective of this study was to transpose the synthesis of
Dropropizine from the usual batch process to flow with the use of capillary
microreactors. The best operation conditions for this synthesis were determined
in the batch process (temperature, reaction time and concentration of the
reaction medium) and in the flow process in the capillary microreactor
(temperature and mean residence time) to maximize the yield of the drug.
Material e métodos
The experimental procedure for batch synthesis of Dropropizine, based on the
patent of Van Lersel (1990), consists of the reaction of Phenylpiperazine and
Glycidol in aqueous medium. In this process two series of tests were performed.
The first series consisted of determining the highest concentration of the
reaction medium so that there was no formation of solids in each reaction
studied. The concentrations tested were 0.4, 0.2 and 0.1 M. Then, the influence
of temperature of 40, 60, 80 and 100 °C on the product yield was verified. In
all assays, aliquots of the reaction medium were collected at times of 0.5, 2,
5, 10 and 20 min for analysis by HPLC-UV. Once the tests were performed in the
batch process, it was transposed to the flow process in microreactors. The
synthesis in the microreactor consisted of preparing two solutions: 1)
Phenylpiperazine (24 mmol; 0.8 M) in water; 2) Glycidol (24 mmol; 0.8 M) in
water. The 2 solutions were fed separately to the microreactor at adequate flow
and temperature. For the flow tests were tested the best experimental condition
determined in the batch process at temperatures of 40, 60, 80 and 100 °C and
mean residence times, τ, 1, 2, 4, 8, 12, 16 and 20 min. The quantification of
the synthesized compounds was performed by high performance liquid
chromatography (HPLC) coupled to UV detector (Shimadzu, mod. Prominence 20AD,
JP), using an eluent composed of phosphate acid buffer solution pH 3:methanol
(88:12, v/v). Flow rate: 0.9 mL/min. The maximum absorbance of Dropropizine
occurs for λ=238 nm
Resultado e discussão
In the tests performed in batch at the concentration of 0.1 M there was
formation of solids, making it impossible to perform the transposition to the
process in flow, since it can compromise the use of this technology through the
blockage of micro channels. There was no formation of solids in the experiments
with concentrations of 0.2 and 0.4 M, and thus the transposition to the flow
process in the microreactors to the concentration of 0.4 M was tested due to the
higher yields observed. Figure 1 shows the results of Dropropizine yield in
batch and in flow, as a function of temperature and mean residence time. The
results presented in Figure 1 show that the yields for the batch tests were
66.8, 68.0 and 90.8% for the mean residence time of 20 min and temperatures of
40, 60 and 80 °C, respectively. In flow and for the same conditions, yields of
78.7, 52.5 and 77.0% were obtained. The yield of 92.8%, the highest observed,
was obtained when the reaction was carried out in flow at 80 °C and mean
residence time 12 min. For the temperature of 100 °C and concentration of
reagents of 0.4 M, the reaction medium presented a different color when compared
to the other experimental conditions, presenting an orange color instead of the
usual yellowish tone, and yields greater than 100%. This observation occurred
both in batch reactions and in those performed in flow. One hypothesis for this
is that there were parallel and/or consecutive reactions whose product has the
same retention time of Dropropizine and also absorbing at the same wavelength.
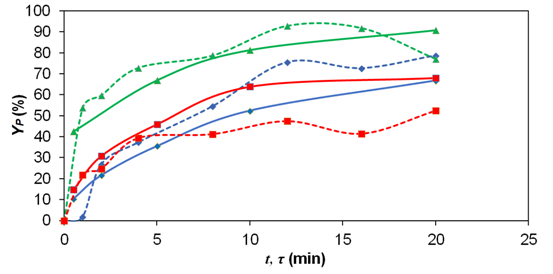
Dropropizine yield (Yp) in batch reactor (__) and in continuous flow (---). Temperature = (•) 40, (■) 60 and (▲) 80 °C; C = 0.4 M of each reagent.
Conclusões
Maximum yield of 92.8% of product was obtained in the flow synthesis of
Dropropizine, from Glycidol and Phenylpiperazine, for concentration of 0.4 M of
each reagent, at 80 °C and mean residence time 12 min. With the sequence of this
study, triplicates will be performed for each of the experimental conditions
studied so far. In addition, efforts will be made to characterize the by-products
formed at 100 °C.
Agradecimentos
We thank the School of Pharmaceutical Science, University of São Paulo - FCF/USP,
São Paulo Research Foundation – FAPESP for the Scientific Initiation Scholarship
n. 2022/01770-3 and my advisor for the all support given.
Referências
JIMÉNEZ-GONZÁLEZ, C.; CONSTABLE, D.J.C.; PONDER, C.S. Evaluating the “Greenness” of chemical processes and products in the pharmaceutical industry—a green metrics primer. Chemical Society Reviews, v. 41, n. 4, p. 1485, 2012.
MACHADO, A. K. M. S.; NEMITZ, M. C.; TODESCHINI, V.; SANGOI, M. S. Characteristics, Properties and Analytical Methods for Determination of Dropropizine and Levodropropizine: A Review. Critical Reviews in Analytical Chemistry, v. 51, n. 2, p .174-182, 2021.
SILVA, R. R. O.; CALVO, P. V. C.; SILVA, M. F., SOLISIO, C.; CONVERTI, A.; PALMA, M. S. A. Flow Synthesis of a Thiazolidine Drug Intermediate in Capillary Microreactors. Chemical Engineering Technology, v. 42, n. 2, p. 465-473, 2019. https://www.researchgate.net/publication/327579603_Flow_Synthesis_of_a_Thiazolizine_Drug_Intermediate_in_Capillary_Microreactor
VAN LERSEL, J. T. M. Preparation of enantiomers of dropropizine Depósito: 22 jun. 1989. Concessão: 3 fev. 1990.
YOSHIDA, J.; TAKAHASHI, Y.; NAGAKI, A. Flash chemistry: flow chemistry that cannot be done in batch. Chemical communications, Cambridge, v. 49, n. 85, p. 896–904, 2013.
















