Autores
Gonzalez Lopez, N.M. (UNIVERSIDAD NACIONAL DE COLOMBIA) ; Guerra Acero, L.M. (UNIVERSIDAD NACIONAL DE COLOMBIA) ; Blanco Medina, I. (UNIVERSIDAD NACIONAL DE COLOMBIA) ; Martinez Ramirez, J.A. (UNIVERSIDAD NACIONAL DE COLOMBIA) ; Garcia Castañeda, J.E. (UNIVERSIDAD NACIONAL DE COLOMBIA) ; Rivera Monroy, Z.J. (UNIVERSIDAD NACIONAL DE COLOMBIA)
Resumo
The peptides Sermorelin (22-29), RGD-20[R] LfcinB (21-25)pal, 26[Nal] LfcinB
(20-30)2, 26[F] LfcinB (20-30)2, were evaluated in serum, plasma, urine, and
cellular culture media, to detect, characterize and quantify each peptide, as
part of the search for an Internal Standard (IS's) for the quantification of
peptides in complex matrices. The pretreatment of the samples was carried out by
means of SPE and PP. The resulting products were evaluated by HPLC-DAD. A unique
profile related to stability of each peptide, in the different matrices, was
established. Sermorelin (22-29) was chosen as the most suitable IS's synthesized
in-house for the quantification of peptides in biological matrices, as it
presented the best stability, physicochemical properties and recovery in all
matrices.
Palavras chaves
Peptides; Biological matrices; Internal Standard
Introdução
In recent years, there has been an important increase in the development of
peptides as pharmaceutical agents for the diagnostic and treatment of diseases
such as cancer, metabolic disorders, among others (D´Aloisio et al, 2021). Their
small size, high specificity, good efficacy, and physicochemical properties
makes them molecules of interest to the pharmaceutical industry for the
development of new drugs.(Wang et al, 2022; Henninot et al, 2018)
Following this growing development of therapeutic peptides, there is an
increasing need for a reliable method for the detection and quantification of
these molecules in complex biological matrices such as urine, plasma, etc. This
aspect has been previously highlighted as an essential tool for drug discovery
(van de Merbel, 2019), to support research in pre-clinical stages (Bronsema et
al, 2012) and has been reported as an analytical challenge for international
agencies such as the World Anti-Doping Agency.(Barroso et al, 2012)
Peptide quantification has been performed using UV, MS, IR, or FL detectors,
especially using chromatographic methodologies, with an appropriate reference
standard (Allenspach et al, 2018). The lack of a reference standard, especially
in the development of novel peptides, makes these works focus on the use of
internal standards (IS´s), mainly to improve the accuracy, precision, and
robustness of the quantification methodology.(Faria et al, 2018)
At present, liquid chromatography has been established as one of the key
methodologies for the identification and quantification of peptides in complex
biological matrices. The present work focuses on a search for an IS´s which
would be suitable for the determination and quantification of peptides in
biological matrices using a conventional HPLC-DAD equipment.
Material e métodos
Four peptides (Figure 1) were selected as possible models to find a
peptide with the ideal physicochemical properties to be used as an IS´s in
peptide quantification assays. As in vivo stability is one of the major
drawbacks of these molecules, peptides from digestion assays (signature
peptides), dimeric peptides, functionalized peptides and peptides with non-
natural motifs were evaluated to establish the most stable and optimal candidate
to be used as IS´s for the quantification of peptide molecules in biological
matrices.
The four peptides Sermorelin (22-29), RGD-Ahx-20[R] LfcinB (21-25)
Pal, 26[Nal] LfcinB (20-30)2, 26[F]
LfcinB (20-30)2 were obtained by means of manual solid-phase peptide
synthesis, using the Fmoc/tBu strategy (SPPS-Fmoc/tBu). The crude product of the
synthesis was purified by means of solid phase extraction (SPE). RP-HPLC
analysis was performed on a Chromolith High Resolution RP-18e Monolithic Column
(50 × 4.6 mm) column.
The four peptides were evaluated in four different biological matrices: serum,
plasma, urine, and in complex biological systems (RPMI culture medium). Peptide
stock solutions (1-3 mg/mL) were added to each complex matrix. An SPE procedure
using Supelco® cartridges (for urine samples), and protein precipitation using
ACN followed by centrifugation (for serum, plasma, and RPMI 1640 Medium) was
used to extract the peptides. The analysis times were variable, depending on the
previously evaluated half-life values for each matrix (data not shown).
Resultado e discussão
The selection of an IS´s is often governed by factors such as availability,
costs, among others. Although methodologies involving MS equipment work with
isotopically labeled IS´s, for routine RP-HPLC analysis it is possible to work
with structural analogue peptides as IS´s that have properties like those of the
analyte of interest, that are resolved in the chromatographic system, and that
are stable under the conditions evaluated. Poor stability in vivo is one of the
biggest drawbacks for the evaluation of peptides in biological matrices, and,
therefore, is an aspect to consider in the quantification of peptides in complex
biological systems.
There are several alternatives to improve the physicochemical, stability and
pharmacokinetic parameters of peptides, among which dimerization and
functionalization (with peptide or non-peptide motifs) stand out. Additionally,
the use of signature peptides, such as those derived from proteolytic digestion
assays or metabolism assays, have been reported as optimal standards for the
analysis of peptides in biological matrices.
The four peptides were evaluated in each matrix for a period of 2 hours to
determine the most optimal peptide to be used as IS's (the most stable and the
one with the best detection/quantification parameters). Sermorelin (22-29) was
the peptide that best met all these conditions, which is why it was established
as the most suitable IS's for the quantification of peptides in biological
matrices. Calibration curves were constructed for each peptide using the IS´s.
The evaluation of the peptides was performed by analyzing each peptide + IS's,
and additionally analyzing a mixture of the 4 peptides. LOD, LOQ, percent
recovery and matrix effect were determined.
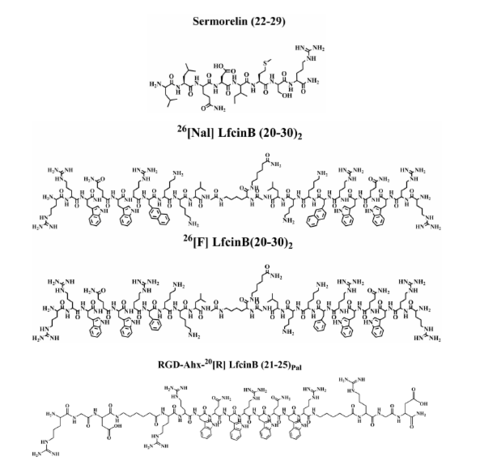
Chemical structures of the 4 peptides used as models to search for a peptide internal standard for quantification assays in biological matrices.
Conclusões
The analytical methodology (HPLC-DAD), and the sample extraction and preparation
processes (SPE and PP) were effective for the analysis of the four peptides, which
allowed establishing a unique profile related to stability of each peptide, in the
different matrices. Sermorelin (22-29) presented the best stability, the most
desirable profile of synthetic and physicochemical properties and the best
percentage recovery in all the matrices, so it was chosen as the most suitable
internal standard synthesized in-house for the quantification of peptides in
biological matrices.
Agradecimentos
To the Ministerio del Deporte and Universidad Nacional de Colombia for their
support in the project: Hermes code 51286.
Referências
ALLENSPACH M.D.; FUCHS J.A.; DORIOT N.; HISS J.A.; SCHNEIDER G.; STEUER C.; Quantification of hydrolyzed peptides and proteins by amino acid fluorescence, Journal of Peptide Science, pp. 1-7 (2018).
BARROSO O.; HANDELSMAN D.J.; STRASBURGER C.; THEVIS M. Analytical Challenges in the Detection of Peptide Hormones for Anti-Doping Purposes, Bioanalysis, 4, pp. 1577-1590 (2012).
BRONSEMA K.J.; BISCHOFF R.; VAN DE MERBEL N.C. Internal standards in the quantitative determination of protein biopharmaceuticals using liquid chromatography coupled to mass spectrometry, Journal of Chromatography B, 893-894, pp. 1-14 (2012).
D´ALOISIO V.; DOGNINI P.; HUTCHEON G.A.; COXON C.R. PepTherDia: database and structural composition analysis of approved peptide therapeutics and diagnostics, Drug Discovery Today, 26, pp. 1409-1419 (2021).
FARIA M.; HALQUIST M.S. Internal Standards for Absolute Quantification of Large Molecules (Proteins) from Biological Matrices by LC-MS/MS. In: Stauffer, M.T., editor. Calibration and Validation of Analytical Methods - A Sampling of Current Approaches. London: IntechOpen; 2018.
HENNINOT A.; COLLINS J.C.; NUSS J.M. The current state of peptide drug discovery: ¿back to the future? Journal of Medicinal Chemistry, 61, pp. 1382–1414 (2018).
VAN DE MERBEL N.C. Protein quantification by LC–MS: a decade of progress through the pages of Bioanalysis, Bioanalysis, 11, pp. 629-644 (2019).
WANG L.; WANG N.; ZHANG W.; CHENG X.; YAN Z.; SHAO G.; WANG X.; WANG R.; FU C. Therapeutic peptides: current applications and future directions, Signal Transduction and Targeted Therapy, 7, pp. 1-27 (2022).
















