Autores
Rubens dos Reis Souza, M. (UNIVERSIDADE TIRADENTES) ; S. Moraes, A. (UNIVERSIDADE TIRADENTES) ; Santos, E. (BROWN UNIVERSITY) ; B. Caramão, E. (UNIVERSIDADE TIRADENTES) ; R. Bjerk, T. (UNIVERSIDADE TIRADENTES) ; C. Krauze, L. (UNIVERSIDADE TIRADENTES)
Resumo
The study aimed to develop a method of extraction of hinokinin from the dried bark of the Commiphora
leptophloeos using the extractor EDGE® and ethanol. Central Composite Design ranging the temperature
(51.7 to 108 °C), and the hold time (1.5 to 8.5 min) was carried out. The analysis of variance showed
statistical significance for the temperature, where the linear and quadratic factors were significant for the
hinokinin GC-MS response, while the linear factor was significant for the mass yield. The better hold time
(6.85 min) and temperature (108 °C) increased the mass yield from 6.4% to 17.9%, and the hinokinin content
from 6.7 to 11.1. The EDGE® method demonstrated good efficiency, use of small sample quantities, low
extraction time, and few sample manipulations.
Palavras chaves
EDGE®; hinokinin; Central composite design
Introdução
Lignans are diphenolic phytoallexins produced by plants that present important pharmacological
properties (Boussetta et al., 2013; Lu et al., 2021; Gusson-Zanetoni et al., 2022). Among them, hinokinin
(C20H18O6) is a dibenzylbutyrolactone lignan described as an “emerging bioactive” (Marcotullio et al.,
2014), which produce exerts anti-inflammatory (Chen et al., 2009), anti-parasitic (Trypanosoma cruzi,
Esperandim et al., 2013), anti-tumoral (SiHa cells, Lima et al., 2022), and anti-viral (human hepatitis B
virus, Huang et al., 2003; human immunodeficiency virus, Cheng et al., 2005; and SARS-CoV, Wen et al.,
2007). Hinokinin also presented predisposing to the development of therapeutic drugs for attention deficit
hyperactivity disorder (ADHD) and anxiety (Timple et al., 2013).
Hinokinin was determined and/or isolated from numerous plant species. It is noteworthy that Yoshiki and
Ishiguro (1933) isolated this compound for the first time from Chamaecyparis obtusa Sieb. et Zucc. (hinoki
wood). Among other species, Commiphora leptophloeos (Mart. – J. B. Gillett) (C. leptophloeos) is a
Brazilian Caatinga medicinal plant used as an alternative against diverse diseases and pathogens (Pereira
et al., 2017; Silva et al., 2019; Pessoa et al., 2021; Dantas-Medeiros et al., 2021; Figueredo et al., 2022). In
this context, more than 300 compounds have been determined in the genus Commiphora (Burseraceae)
such as terpenoids (monoterpenoids, sesquiterpenoids, diterpenoids, and triterpenoids), reducing sugars,
steroids, carbohydrates, long-chain aliphatic derivatives, and phenolic (tannins, flavonoids, acids, and
lignans) (Shen et al., 2012). Hinokinin was extracted by saturation in chloroform (250 mL and 24 h) from C.
leptophloeos dried bark and identified for the first time in genus Commiphora via spectrum 1H NMR after
fractionation (Pereira et al., 2017). These extracts showed several activities against Gram-negatives,
Gram-positives (for example S. aureus), and fungi; as well as against Mycobacterium (M. smegmatis and
M. tuberculosis) (Pereira et al., 2017), which may be related to the presence of this lignan.
Nodaways, the research of extraction analytical methods has focused on principles of green analytical
chemistry (GAC), which corresponds to the improvement of the quality of human life based on
sustainability economic, social, and environmental (Płotka-Wasylka et al., 2021). Therefore, there is a
necessity for safer analytical methods to present low energy consumption, using less-toxic solvents,
favoring substances based on renewable sources, and avoiding derivatization (Płotka-Wasylka et al.,
2021). In this context, miniaturized methods and the use of small sample quantities and green solvents,
allied to low extraction time and few sample manipulations, tend to be chosen. C.E.M Corporation (2017)
developed the Energized Dispersive Guided Extraction – EDGE® extractor, describing it as “faster than
Soxhlet, more automated than QuEChERS, and simpler than other solvent extraction systems”. EDGE® may
reduce of significate form the time extraction, solvent consumption, and steps in the extraction of organic
compounds for diverse matrixes, including sediment, soil, and plants (C.E.M Corporation, 2017). Kinross et
al. (2020) showed a description of EDGE® system operation that combines Pressurized Liquid Extraction
(PLE) and dispersive Solid Phase Extraction (dSPE), although the use of dispersive solid phase is optional.
For more information about the EDGE® system, readers can consult the equipment literature (C.E.M
Corporation, 2017) or the CEM website (https://cem.com/edge/).
There are few works in the literature using the EDGE® system. Based on these facts, the aims of this study
are: (1) to develop an efficient extraction method of hinokinin from C. leptophloeos dried bark using
EDGE® extractor and Central composite design; (2) to use EDGE® extractor as a viable alternative to
obtain extracts riches in secondary metabolites from C. leptophloeos bark. It is noteworthy that this is the
first work using EDGE® as a secondary metabolites extractor in medicinal plants.
Material e métodos
C. leptophloeos bark was collected in the settlement “Barra da Onça”, Poço Redondo – SE (9°48'25,6"S
37°38'12,9"W), Northeast Brazil. Ethanol was the solvent chosen to develop the Central Composite Design
(CCD) using C. leptophloeos bark (2 g, 32-60 mesh), cellulose C9-G1-C9 (sandwich) Q-Disks; with 10 mL
of solvent at top volume, and 5 mL of solvent at bottom volume. These extracts were prepared by dilution
of 6.0 µL of internal standard p-terphenyl-d14 (50 µg mL-1) and 30.0 µL of each extract into DCM (final
volume 400 µL) and injected in GC-MS (SIM mode). The better EDGE® condition was also carried out in
triplicate in GC-MS (SIM mode). All analyses were performed using gas chromatography coupled to a
mass spectrometry (GCMS-QP2010plus, Shimadzu - Kyoto, Japan) with electron ionization (EI, 70 eV)
mode; equipped with an AOC-20i automatic injector (Shimadzu, Japan) and ZB-5 column (5% phenyl 99%
dimethylpolysiloxane; Zebron, Torrance, CA, USA; 30 m, 0.25 mm ID and 0.25 μm film thickness). Injector,
interface, and ionization source temperatures were maintained at 280.0 ºC, 300.0 ºC, and 280.0 ºC,
respectively. The helium gas flow (99.9995%) was 0.8 mL min-1. For the Scan mode (45-400 m/z): The
initial oven temperature program was 60.0 °C (hold time of 1.0 min); heated to 210.0 °C at 5.0 °C min-1;
heated to 280.0 °C at 10.0 °C min-1 (hold time of 15.0 min). The injection volume was 1.0 µL in splitless
mode. For the SIM: The oven was heated from 200.0 °C (hold time of 4.0 min) and heated to 300.0 °C at
10.0 °C min-1 (hold of 11.0 min). Injection volume was 1.0 µL in split mode 1:10. In this sense, for CCD, the
ethanolic extract re-dissolved into DCM was injected in the GC-MS (SCAN mode) and, retention time (tR),
quantification ion (QI, m/z), and confirmation ions (CI, m/z) were selected of the target compounds
hinokinin (QI = 135; CI = 136 and 354) and p-terphenyl-d14 (QI = 244; CI = 243). The results were
obtained from ratio = hinokinin area/p-terphenyl-d14 area. In this study, response surface methodology
(RSM), central composite design (CCD), and desirability function were used to optimize the mass yield and
hinokinin GC-MS response in bark samples. The low, middle, and high levels for each variable were
designated as -1, 0, and 1, respectively. The central point (n = 5) was carried out to measure the
experiment error. A total of 13 experiments were performed to analyze the factors hold time and
extraction temperature in an EDGE®. The desirability function is a technique normally used to determine
the optimum factor levels that ensure the conformity of all the responses involved in the process,
concomitantly, the finest union value of the desirable responses (Candioti et al., 2014; Hazir et al., 2021).
This function presents several values between 0 and 1, where values close to 1 are interpreted as desirable
responses and values close to 0 can be understood as outside the acceptable range or unacceptable
response.
Resultado e discussão
The responses of the model were expressed as a mass yield of each experiment and ratio of hinokinin
area/ p-terphenyl-d14 area, to determine the best EDGE® extraction condition. It is worth mentioning that
different from the accelerated solvent extraction (ASE) system that combines elevated temperatures and
pressures with liquid solvents (Richter et al., 1996), the EDGE® extractor does not allow selecting a
specific pressure. Therefore, the programmable parameters include temperature, hold time, and volume. In
this sense, this work fixed the extraction volume, since a sufficient volume of solvent is needed only to
completely cover the entire sample; and varied temperature and hold time, to know whether the variation
of these parameters influences the extraction of hinokinin, maintaining a good extraction yield.
Established on this information, our research was to optimize the affecting variables in the extraction
process by central composite design (2ˆ2). The analysis of variance (ANOVA) showed statistical
significance for the temperature parameter, where the linear and quadratic factors were significant for the
hinokinin GC-MS response. In contrast, the linear factor was significant for the yield. This means that hold
time and second-order interactions between factors are unimportant for this study and should not be
considered. The quality of the fit evaluation to the second-order model was assured based on the Rˆ2,
which were 0.9381 and 0.9629 for yield and hinokinin response, respectively. In addition, the model fitness
was determined through the lack-of-fit test (p < 0.05), this result indicates the model is suitable to
accurately predict the variation.
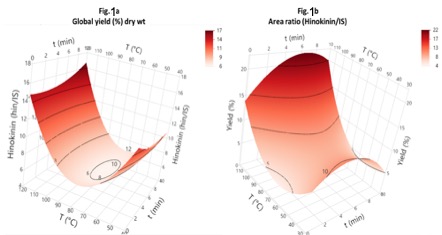
It was possible to see the temperature effect when this parameter increased from 60 °C to 108 °C (Figure
1a), there was an increase in the mass yield percentage from 6.4% to 17.6%, and hinokinin content with the
peak ratio increased from 6.7 to 10.9 (Figure 1b), respectively. In this sense, the increase in temperature
resulted in a steady decrease in the density, surface tension, and solvent viscosity, which allow enhanced
diffusivities (Lee and Kim, 2010; Pereira et al., 2016). This process improves the penetration of solvent into
the sample, and the conditions for the extraction, solubilization, and dissolve more rapidly of the
compounds (Lee and Kim, 2010; Santos et al., 2019).
The increase of total polyphenols content and lignans was presented when the extraction for High Voltage
Electrical Discharges (HVED) was used with 0 kV, ethanol (100%), and increased temperature from 20 °C
to 60 °C (Boussetta et al., 2013). Similar results were determined by Lee and Kim (2010) and Pereira et al.
(2016) using pressurized liquid extraction in the extraction of lignans, and by Santos et al. (2019)
extracting α-bisabolol from candeia (Eremanthus erythropappus) wood. It is noteworthy that the extract
obtained at 108 °C showed different colors (olive color), due probably to the degradation of chlorophyll
(Pereira et al., 2016).
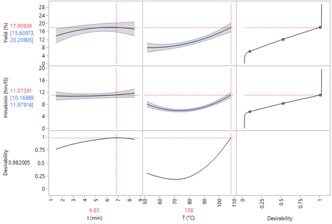
The individual and global desirability functions were applied to determine the optimum condition
extraction. Two-dimension plots of the global desirability obtained are shown in Fig. 3. The software
achieved accurately the best condition for each variable, even though hold time was not significant. The
dotted lines show the current variable settings and response values, and the curves are the 95%
confidence intervals for each profile. The optimal condition was obtained at a time of 6.85 min and the
temperature was 108 °C. At this operating condition, it is predicted that the maximum yield percentage
was 17.9%, varying between 15.6 to 20.2%. In another hand, the hinokinin recovery was an 11.1 peak ratio,
with a variation between 10.2 and 12.0 peak ratio. These values were obtained with the overall desirability
of 0.982005.
For confirmation of the better condition determined (6.85 min and 108 °C), a triplicate was carried out.
The results presented an average yield of 21.4% ± 1.2 (%RSD = 5.5) and a peak ratio of 12 ± 1.3 (%RSD =
10.9), which corroborated with the statistic model shown before. This result may be considered better than
the showed by Pereira et al. (2017), in which the %yield ranged between 9.8% and 12.3%, using the
solvents cyclohexane (9.8%), chloroform (10.2%), ethyl acetate (10.5%), methanol (11.8%), and aqueous
(12.3%). On the other hand, the ethanolic extract used in the biological tests developed by Silva et al.
(2019) presented a similar result (23.7%), which corroborates the choice of this solvent. However, these
works were characterized by the use of a high quantity of samples, 25 g (Pereira et al., 2017) and 446 g
(Silva et al., 2019), as well as used exhaustive extraction methods with 24h and 72h, respectively.
The results also showed a difference in extraction time and hinokinin content by EDGE® when compared
with other extraction methods. In this sense, the areas (%) of hinokinin determined in the ethanolic
extracts were 12.06% (ultrasound), followed by 18.30% (Soxhlet), and 50.75% (EDGE®), whereas the DCM
extracted 21.72% by ultrasound, 6.41% (Soxhlet), and 48.21% (EDGE®). It is worth mentioning that the
sample mass used in these methods was 20 g, demonstrating that the use of only 2 g by EDGE® led to
better analytical efficiency.
Conclusões
A method for extraction of hinokinin from C. leptophloeos dried bark using an EDGE® was developed. The
results showed that EDGE® such as a viable alternative to obtain extracts riches in secondary metabolites,
using a small number of samples, low extraction time, few sample manipulations, and use of a non-toxic
solvent, which represented better efficiency in total mass yield and hinokinin content than other extraction
methods. Furthermore, the use of GC-MS allowed us to separate and identify for the first time the
compounds never identified in this part of the plant, mainly hinokinin. Therefore, the use of EDGE®
represented what may be again to the industry in the production of herbal medicinal products, using a
method focused on principles of the GAC. Future works should be published and biological tests have shown.
Agradecimentos
The authors are grateful to PNPD/CAPES (Process number: 88887.357888/2019-00), FAPITEC/SE, and CNPq.
Referências
Bezerra, M.A., Santelli, R.E., Oliveira, E.P., Villar, L.S., Escaleira, L.A., 2008. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76, 965–977. https://doi.org/10.1016/j.talanta.2008.05.019
Bharath, B., Perinbam, K., Devanesan, S., AlSalhi, M.S., Saravanan, M., 2021. Evaluation of the anticancer potential of Hexadecanoic acid from brown algae Turbinaria ornata on HT–29 colon cancer cells. J. Mol. Struct. 1235, 130229. https://doi.org/10.1016/j.molstruc.2021.130229
Borges, A., Casoti, R., Silva, M.L.A., Cunha, N.L., Pissurno, A.P.R., Kawano, D.F.R., Laurentiz, R.S., 2018. COX inhibition profiles and molecular docking studies of the lignan hinokinin and some synthetic derivatives. Mol. Inform. 37(12), 1800037. https://doi.org/10.1002/minf.201800037
Boussetta, N., Turk, M., Taeye, C.D., Larondelle, Y., Lanoisellé, J.L., Vorobiev, E., 2013. Effect of high voltage electrical discharges, heating, and ethanol concentration on the extraction of total polyphenols and lignans from flaxseed cake. Ind. Crops Prod. 49, 690–696. http://dx.doi.org/10.1016/j.indcrop.2013.06.004
C.E.M Corporation, Application Notes, (2017). http://cem.com/en/literature? application=Extraction&literature _ type=Application _ Notes (accessed December, 2021).
Candioti, L.V., Zan, M.M.D., Cámara, M.S., Goicoechea, H.C., 2014. Experimental design and multiple response optimization. Using the desirability function in analytical methods development. Talanta 124, 123–138. http://dx.doi.org/10.1016/j.talanta.2014.01.034
Carlis, M.S.P., Féboli, A., Laurentiz, A.C., Filardi, R.S., Oliveira, A.H.P., Silva, M.L.A., Anjos, L.A., Magalhães, L.G.M., Laurentiz, R.S., 2019. In vitro anthelmintic activity of the crude hydroalcoholic extract of Piper cubeba fruits and isolated natural products against gastrointestinal nematodes in sheep. Vet. Parasitol. 275, 108932. https://doi.org/10.1016/j.vetpar.2019.108932
Carneiro, C.N., Gomez, F.J.V., Spisso, A., Silva, M.F., Azcarate, S.M., Dias, F.S, 2020. Geographical characterization of South America wines based on their phenolic and melatonin composition: An exploratory analysis. Microchem. J. 158, 105240. https://doi.org/10.1016/j.microc.2020.105240
Chen, J.J., Chung, C.Y., Hwang, T.L., Chen, J.F., 2009. Amides and benzenoids from Zanthoxylum ailanthoides with inhibitory activity on superoxide generation and elastase release by neutrophils. J. Nat. Prod. 72, 107–111. 10.1021/np800689b
Cheng, M.J., Lee, K.H., Tsai, I.L., Chen, I.S., 2005. Two new sesquiterpenoids and anti-HIV principles from the root bark of Zanthoxylum ailanthoides. Bioorg. Med. Chem. 13, 5915–5920. 10.1016/j.bmc.2005.07.050
Dantas-Medeiros, R., Furtado, A.A., Zanatta, A.C., Torres-Rêgo, M., Lourenço, E.M.G., Alves, J.S.F., Galinari, É., Rocha, H.A.O., Guerra, G.C.B., Vilegas, W., Araújo, T.A.S., Fernandes-Pedrosa, M.F., Zucolotto, S.M., 2021. Mass spectrometry characterization of Commiphora leptophloeos leaf extract and preclinical evaluation of toxicity and anti-inflammatory potential effect. J. Ethnopharmacol. 264, 113229. https://doi.org/10.1016/j.jep.2020.113229
Es’haghia, Z., Ebrahimi, M., Hosseini, M-S., 2011. Optimization of a novel method for determination of benzene, toluene, ethylbenzene, and xylenes in hair and waste water samples by carbon nanotubes reinforced sol–gel based hollow fiber solid phase microextraction and gas chromatography using factorial experimental design. J. Chromatogr. A 1218, 3400–3406. https://doi.org/10.1016/j.chroma.2011.03.043
Esperandim, V.R., da, S.F.D., Saraiva, J., Silva, M.L.A., Costa, E.S., Pereira, A.C., Bastos, J.K., de, A.S., 2010. Reduction of parasitism tissue by treatment of mice chronically infected with Trypanosoma cruzi with lignano lactones. Parasitol. Res. 107, 525–530. 10.1007/s00436-010-1885-z
Figueredo, F.G., Cabrera, S.P., Bispo, M.A.L., Sarmento, S.T.M., Filho, J.R., Freitas, P.R., Silva, M.J.W., Pereira, V.S., Ferreira, M.E.F., Melo, C.H.D., Fonteles, M.M.F., 2022. UPLC/QTOF-MS/MS analysis and antibacterial activity of Commiphora leptophloeos (Mart.) J. B. Gillett against multi-drug resistant Staphylococcus aureus and Pseudomonas aeruginosa. J. Herb. Med., 100506. https://doi.org/10.1016/j.hermed.2021.100506
Gusson-Zanetoni, J.P., Silva, J.S.G.M., Picão, T.B., Cardin, L.T., Prates, J., S.O. Sousa, T. Henrique, S.M. Oliani, E.H. Tajara, M.L.A. Silva, N.L. Cunha, A.C.S. Gomes, R.S. Laurentiz, F.C. Rodrigues-Lisoni. Effect of Piper cubeba total extract and isolated lignans on head and neck cancer cell lines and normal fibroblastos. J. Pharm. Sci. 148 (2022) 93–102. https://doi.org/10.1016/j.jphs.2021.09.002
Hazir, E., Seker, S., Koc, K.H., Dilik, T., Erdinler, E.S., Ozturk, E, 2021. Optimization of plasma treatment parameters to improve the wood-coating adhesion strength using Taguchi integrated desirability function approach. J. Adhes. Sci. Technol. 35, 451–467. https://doi.org/10.1080/01694243.2020.1816668
Huang, R.L., Huang, Y.L., Ou, J.C., Chen, C.C., Hsu, F.L., Chang, C., 2003. Screening of 25 compounds isolated from Phyllanthus species for anti-human Hepatitis B virus in vitro. Phytother. Res. 17, 449–453. 10.1002/ptr.1167
Khuri, A.I., Mukhopadhyay, S., 2010. Resp. Surf. Methodol. 2, 128–149. https://doi.org/10.1002/wics.73
Kinross, A.D., Hageman, K.J., Doucette, W.J., Foster, A.L., 2020. Comparison of Accelerated Solvent Extraction (ASE) and Energized Dispersive Guided Extraction (EDGE) for the analysis of pesticides in leaves. J. Chromatogr. A 1627, 461414. https://doi.org/10.1016/j.chroma.2020.461414
Lee, H.J., Kim, C.Y., 2010. Simultaneous determination of nine lignans using pressurized liquid extraction and HPLC-DAD in the fruits of Schisandra chinensis. Food Chem. 120, 1224–1228. 10.1016/j.foodchem.2009.11.068
Lima, L.M., Perazzo, F.F., Carvalho, J.C.T., Bastos, J.K., 2007. Anti-inflammatory and analgesic activities of the ethanolic extracts from Zanthoxylum riedelianum (Rutaceae) leaves and stem bark. J. Pharm. Pharmacol. 59, 1151–1158. 10.1211/jpp.59.8.0014
Lima, R.G., Lisoni, F.C.R., Picão, T., Santos, F., Orenha, R.P., Borges, A., Molina, E., Parreira, R.L.T., M.L.A.E. Silva, M. Santos, R.S. Laurentiz, 2021. In vitro and in silico cytotoxicity of hinokinin-loaded PLGA microparticle systems against tumoral SiHa cells. Nat. Prod. Res., 1-8. https://doi.org/10.1080/14786419.2021.2000409
Lima, T.C.; Lucarini, R.; Volpe, A.C.; Andrade, C.Q.; Souza, A.M.; Pauletti, P.M.; Januário, A.H.; Símaro, G.V.; Bastos, J.K.; Cunha, W.R.; Borges.A., 2017, In vivo and in silico anti-inflammatory mechanism of action of the semisynthetic (–)-cubebin derivatives (–)-hinokinin and (–)-O-benzylcubebin. Bioorg. Med. Chem. Lett. 27(2), 176–179. https://doi.org/10.1016/j.bmcl.2016.11.081
Lu, Q., Zheng, R., Zhu, P., Bian, J., Liu, Z., Du. J., 2021. Hinokinin alleviates high-fat diet/streptozotocin-induced cardiac injury in mice through modulation in oxidative stress, inflammation, and apoptosis. Biomed. Pharmacother. 137, 111361. https://doi.org/10.1016/j.biopha.2021.111361
Marcotullio, M.C., Pelosi, A., Curin, M., 2014. Hinokinin, an Emerging Bioactive Lignan. Molecules 19, 14862–14878. 10.3390/molecules190914862
Palsikowski, P.A., Besen, L.M., Klein, E.J., Silva, C., Silva, E.A., 2019. Optimization of ultrasound-assisted extraction of bioactive compounds from B. forficata subsp. Pruinosa. Can. J. Chem. Eng., 1–13. https://doi.org/10.1002/cjce.23757
Pereira, J.J.S., Pereira, A.P.C., Jandú, J.J.B., Paz, J.A., Crovella, S., Correia, M.T.S., Silva, J.A., 2017. Commiphora leptophloeos Phytochemical and Antimicrobial Characterization. Front. Microbiol. 8(52), 1–10. 10.3389/fmicb.2017.00052
Pereira, R.G., Garcia, V.L., Rodrigues, M.V.N., Martínez, J., 2016. Extraction of lignans from Phyllanthus amarus Schum. & Thonn using pressurized liquids and low pressure methods. Sep. Purif. Technol. 158, 204–211. http://dx.doi.org/10.1016/j.seppur.2015.12.007
Pessoa, R.F., I.A.D. Figueiredo, S.R.D. Ferreira, A.R.L.F.C. Silva, R.L.M. Paiva, L.V. Cordeiro, E.O. Lima, S.P. Cabrera, T.M.S. Silva, F.A. Cavalcante. Investigation of ethnomedicinal use of Commiphora leptophloeos (Mart.) J. B. Gillett (Burseraceae) in treatment of diarrhea J. Ethnopharmacol. 268 (2021) 113564. https://doi.org/10.1016/j.jep.2020.113564
Płotka-Wasylka, J., Mohamed, H.M., Kurowska-Susdorf, A., Dewani, R., Fares, M.Y., Andruch, V., 2021. Green analytical chemistry is an integral part of sustainable education development. Curr. Opin. Green Sustain. Chem. 31, 100508. https://doi.org/10.1016/j.cogsc.2021.100508
Richter, B.E., Jones, B.A., Ezzell, J.L., Porter, N.L., Avdalovic, N., Pohl, C., 1996. Accelerated Solvent Extraction: A Technique for Sample Preparation. Anal. Chem. 68, 1033–1039. https://doi.org/10.1021/ac9508199
Santos, K.A., Gonçalves, J.E, Cardozo-Filho, L., Silva, E.A., 2019. Pressurized liquid and ultrasound-assisted extraction of α-bisabolol from candeia (Eremanthus erythropappus) wood. Ind. Crops Prod. 130, 428–435. https://doi.org/10.1016/j.indcrop.2019.01.013
Shen, T., Li, G-H., Wang, X-N., Lou, H-X., 2012. The genus Commiphora: A review of its traditional uses, phytochemistry, and pharmacology. J. Ethnopharmacol. 142, 319–330. https://doi.org/10.1016/j.jep.2012.05.025
Silva, I.F., Guimarães, A.L., Amorim, V.S., Silva, T.M.G., Peixoto, R.M., Nunes, X.P., Silva, T.M.S., Costa, M.M., 2019. Antimicrobial activity of ethanolic extracts from Commiphora leptophloeos (Mart.) J.B. Gillett against Staphylococcus spp. isolated from cases of mastitis in ruminants. Ciênc. Anim. Bras. 20, 1–14. https://doi.org/10.1590/1089-6891v20e-57228
Timple, J.M.V., Magalhães, L.G., Rezende, K.C.S., Pereira, A.C., Cunha, W.R., Silva, M.L.A., Mortensen, O.V., Fontana, A.C.K., 2013. The Lignan (−)-Hinokinin Displays Modulatory Effects on Human Monoamine and GABA Transporter Activities. J. Nat. Prod. 76, 1889–1895. https://doi.org/10.1021/np400452n
Wen, C-C., Kuo, Y-H., Jan, J-T., Liang, P-H., Wang, S-Y., Liu, H-G., Lee, C-K., Chang, S-T., Kuo, C-J., Lee, S-S., Hou, C-C., Hsiao, P-W., Chien, S-C., Shyur, L-F., Yang, N-S., 2007. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 50, 4087–4095. https://doi.org/10.1021/jm070295s
Yoshiki, Y.; Ishiguro, T., 1933, Crystalline constituents of hinoki oil. Yakugaku Zasshi. 53, 73–151. Google Scholar
















