Autores
Sarria-villa, R. (UNIVERSIDAD DEL CAUCA) ; Gallo, J.A. (UNIVERSIDAD DEL CAUCA) ; Benitez, R. (UNIVERSIDAD DEL CAUCA) ; Pabon, S. (UNIVERSIDAD DEL CAUCA) ; Cuetocue, M. (UNIVERSIDAD DEL CAUCA)
Resumo
Water scarcity and contamination are two undeniable global problems and
significant change has to be addressed. Adsorption is one of the most used methods
for the treatment of polluted water due to its effectiveness and ease of use.
Pinus patula bark and Eucalyptus grandis leaves were used to make adsorbents for
gold and mercury, respectively, present in water sources. The adsorbent for gold
was prepared by crosslinking the tannins present in the bark of Pinus patula. The
BET analysis showed a surface area of 4.172 m2g-1 and percentage of Au adsorption
of 98-99%. Iron nanoparticles were synthesized using an aqueous extract of
Eucalyptus grandis and iron salts. BET analysis indicated a surface area of 131.90
m2g-1 and the synthesis yield of the nanoparticle was 98%.
Palavras chaves
Adsorption; Forest residues; Heavy metals
Introdução
The growing demand for gold makes it crucial to recover gold from waste products
that inevitably increase. The Colombian government included gold mining as one
of the engines of development for the period 2014-2018. This expansion of gold
mining activities can have considerable consequences for the environment, as it
can lead to further pollution and environmental degradation. This makes the need
to control emissions much more intense and the restrictions placed on waste
disposal and environmental regulations much more stringent (Syed, 2012). Methods
such as precipitation, ion exchange, solvent extraction, and flotation are
available for mercury recovery, but these methods have significant drawbacks
such as the use of toxic chemicals, high reagent requirements, and the
generation of secondary waste, toxic that require disposal. A better alternative
in this regard is to develop environmentally friendly and cost-effective
lyosorption techniques that use biomass to recover metal from waste (Choudhary
et al., 2018). Tannins are well known to be inexpensive and ubiquitous natural
polymers that can be easily extracted from plants. Adsorption is a highly
efficient, economical and widely used method for the uptake of metal ions from
different aqueous solutions (Yin et al., 2013). In particular, in Colombia the
bark of Pinus patula is an abundant and cheap forest residue that can be used as
an alternative for the adsorption of metal ions. In addition, the use of biomass
as a component in the synthesis of nanoparticles has a quite remarkable
advantage at the level of green chemistry by reducing the need for reagents to
obtain nanoparticles, in addition to the fact that biomass can perform several
functions throughout the synthesis, from a complexing system, to being part of
the stabilizing effect of the nucleus (García, 2015). Therefore, it is necessary
to optimize the synthesis of nanoparticles using aqueous extracts of Eucalyptus
grandis foliage and to explore their possible use as active adsorbent material
for mercury retention.
Material e métodos
Samples and reagents
Samples of Pinus patula bark were acquired from the company Smurfit Carton de
Colombia, located in the municipality of Sotará Cauca-Colombia. All reagents and
chemicals used in this study were of analytical quality. A standard solution of
1000 mg.L-1 of Cu(II), Fe(III), Zn(II), Ni(II), Au(III) and Hg(II), MERCK brand
was used. The foliage sample of Eucalyptus grandis was obtained from the forest
of the Cooperativa Agroforestal del Cauca (COOTRAFORC), located in Vereda
Gonzales-Popayán-Cauca.
Preparation of the adsorbent
50 and 150 g of Pinus patula bark previously ground and sieved, and aggregate
300 to 600 mL of deionized water and between 3.0 and 7.0 g of NaOH, the mixture
was stirred at 90°C. Subsequently, it was filtered and the liquid fraction was
dried and mixed with 20 mL of sulfuric acid and stirred for 12 h flush at 95°C.
For obtaining nanoparticles, Eucalyptus grandis foliage was mixed with deionized
water and heated to 80°C for 5 minutes, subsequently, it is vacuum filtered and
centrifuged at 1250 rpm for 5 minutes, the extract is completed with water and
stored at room temperature (Huang et al., 2010). Iron salts were mixed, heated
with constant stirring and after 5 minutes 20 mL of a sodium hydroxide solution
(NaOH) were added with controlled stirring.
Adsorption tests
Adsorption tests to tannin adsorbent varying concentrations of HCl (0.1-5.0
mol.L-1) were carried out in triplicate mixing 10 mL of test solutions
containing individual metals Cu(II), Fe(III), Zn(II), Ni(II) and Au(III), each
metal at a concentration of 100 mg.L-1 with 10 mg of dry adsorbent, the samples
were shaken for 24 h until equilibrium was reached. Tests to evaluate Au(III)
adsorption isotherms were carried out by shaking 10 mg of the dry adsorbent
together with 10 mL of test solutions containing 50-700 mg.L-1 of Au(III) in
HCl 1.0 M for 48h at a temperature of 30°C.
Adsorbents characterization
Tannin and nanoparticles adsorbents were characterized by instrumental analysis
using Transmission Electron Microscopy (TEM), FT-IR spectroscopy and surface
area analysis using the BET technique.
Resultado e discussão
The process of crosslinking the tannins extracted from the bark, when
concentrated sulfuric acid is used as a crosslinking agent, leads to the
formation of a rigid material with adequate porosity and particle size for
adsorption processes (Gurung et al., 2011). That is why the IR spectra of
the tannins, were analyzed once the crosslinking was carried out. The tannins
presented a moderate band between 3400 and 3600 cm-1 corresponding to the
stretching vibrations of the O-H bond of phenolic compounds, characteristic of
tannins, as well as characteristic bands of C-H stretching at 2900 cm-1, the IR
spectrum of the tannins also showed the absorption band at 1384.12 cm-1 that are
assigned for the O-H bending, the peak of 1638.52 cm-1 that is assigned to the
stretching vibration of the C=C bonds of the aromatic ring. These
results indicate that the functional groups of the tannins underwent a
modification in the structure due to crosslinking, when treated
with acid, hydrolysis or self-condensation can happen. The results
obtained by BET showed an area of 4.17 m2/g to tannin adsorbent. Scanning
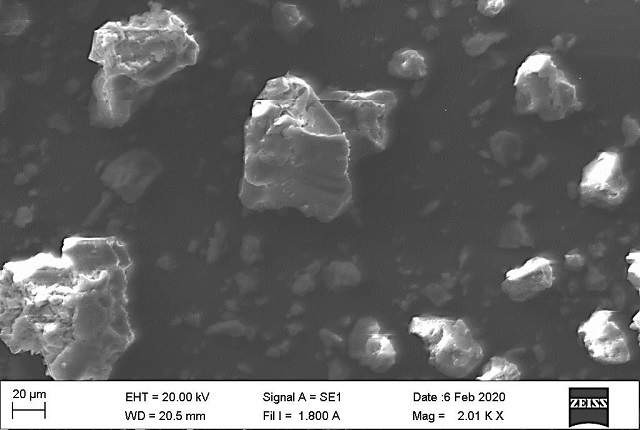
electron microscopy (SEM) was used to determine the particle size. In figure 1
it can be seen that the particle size for the adsorbent obtained is in the range
of 20 microns.
For the synthesis of nanoparticles with a NaOH concentration of 1.7 M,
temperatures from 87 °C, extract volume close to 1 mL, a yield percentage of
approximately 96% is obtained. Triplicate tests of these points were carried
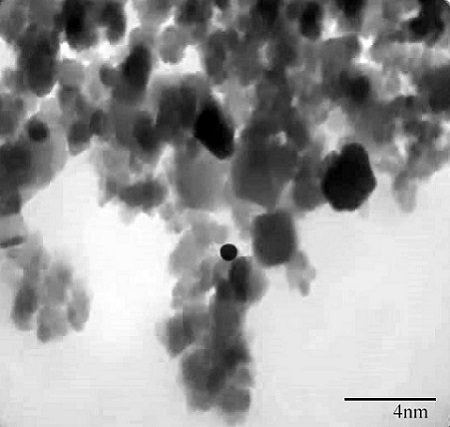
out, obtaining an average yield of 98.99% ± 0.21. To determine the size of the
nanoparticle, transmission electron microscopy (TEM) was used with an average
nanoparticle size of approximately 8.97 nm, which is an average size close to
that exposed by Alvear et al., 2017 of 7 nm and that of Awwad and Salem, 2013 of
8 nm, Fig. 2 shows the micrograph obtained for the synthesized particle in the
nanometer range.
Conclusões
By crosslinking the tannins with concentrated sulfuric acid extracted from
the bark of Pinus patula, the prepared adsorbent exhibited high selectivity and
high adsorption capacity for Au(III) in a low concentration range of HCl
solutions. Iron oxide nanoparticle prepared from an aqueous extract of Eucalyptus
grandis foliage, due to its chemical stability, as well as the nature of the
biomass and its low cost, in addition to its great estimated surface area, is
presented as an alternative for obtaining this type of material.
Agradecimentos
The authors thank to group GIQA, group QPN, Vicerrectoría de Investigaciones-
Universidad del Cauca, Department of Chemistry of the Universidad Del Cauca
(501100005682) for their collaboration in the development of this research.
Referências
S. SYED. Recovery of gold from secondary sources—A review. Hydrometallurgy, vol. 115–116, pp. 30–51, Mar. 2012.
B. BETANCUR-CORREDOR, J. C. LOAIZA-USUGA, M. DENICH, AND C. BORGEMEISTER. Gold mining as a potential driver of development in Colombia: Challenges and opportunities. J. Clean. Prod., vol. 199, pp. 538–553, Oct. 2018.
B. C. CHOUDHARY, D. PAUL, A. U. BORSE, AND D. J. GAROLE. Surface functionalized biomass for adsorption and recovery of gold from electronic scrap and refinery wastewater. Sep. Purif. Technol., vol. 195, pp. 260–270, 2018. https://doi.org/10.1016/j.seppur.2017.12.024.
P. YIN, M. XU, R. QU, H. CHEN, X. LIU, J. ZHANG, AND Q. XU. Uptake of gold (III) from waste gold solution onto biomass-based adsorbents organophosphonic acid functionalized spent buckwheat hulls. Bioresour. Technol., vol. 128, pp. 36–43, Jan. 2013.
D. E. GARCÍA, W. G. GLASSER, A. PIZZI, S. PACZKOWSKI, AND M.-P. LABORIE. Hydroxypropyl tannin from Pinus pinaster bark as polyol source in urethane chemistry. Eur. Polym. J., vol. 67, pp. 152–165, Jun. 2015.
I. N. GARCÍA. Síntesis verde de nanopartículas para la eliminación de colorantes en medios acuosos,” Universidad de la Coruña, 2015.
K. ALEGRIA. Determinación de la capacidad de adsorción de la resina de Pinus Patula modificada químicamente para retener cromo de efluentes generados por la actividad de curtiembres,” Universidad del cauca, 2017.
X. HUANG, Y. WANG, X. LIAO, AND B. SHI. Adsorptive recovery of Au3+ from aqueous solutions using bayberry tannin-immobilized mesoporous silica. J. Hazard. Mater., vol. 183, no. 1–3, pp. 793–798, Nov. 2010.
M. GURUNG, B. B. ADHIKARI, H. KAWAKITA, K. OHTO, K. INOUE, S. ALAM. Recovery of Au(III) by using low cost adsorbent prepared from persimmon tannin extract, Chem. Eng. J. 174, 556-563, 2011. https://doi.org/10.1016/j.cej.2011.09.039.
G. TONDI, A. PETUTSCHNIGG. Middle infrared (ATR FT-MIR) characterization of industrial tannin extracts, Ind. Crops Prod. 65, 422-428, 2015. https://doi.org/10.1016/j.indcrop.2014.11.005.
T. OGATA AND Y. N. RESEARCH. Mechanisms of gold recovery from aqueous solutions using a novel tannin gel adsorbent synthesized from natural condensed tannin, Water Res. 39, 4281-4286, 2005. https://doi.org/10.1016/j.watres.2005.06.036.
M. E. ROSÁNGELES HERNÁNDEZ, P. CARRASCO, R. MUJICA. Evaluación de la capacidad de adsorción de desechos agroindustriales para la remoción de ácido acético, Rev. Fac. Ing. UCV. 22 (2007) http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0798- 40652007000300004&lng=es&nrm=iso
F.C. WANG, JM. ZHAO, WK. WANG, HZ. LIU. Superior Au-adsorption performance of aminothiourea-modified waste cellulosic biomass. J. Cent. South Univ. 25, 2992-3003, 2018. https://doi.org/10.1007/s11771-018-3969-3.
F. LIU, G. PENG, T. LI, G. YU, S. DENG. Au(III) adsorption and reduction to gold particles on cost- effective tannin acid immobilized dialdehyde corn starch, Chem. Eng. J. 370, 228-236, 2019. https://doi.org/10.1016/j.cej.2019.03.208.
F. LIU, S. WANG, S. CHEN. Adsorption behavior of Au(III) and Pd(II) on persimmon tannin functionalized viscose fiber and the mechanism, Int. J. Biol. Macromol.152, 1242-1251, 2020. https://doi.org/10.1016/j.ijbiomac.2019.10.221.
M. GURUNG, B. B. ADHIKARI, H. KAWAKITA, K. OHTO, K. INOUE, S. ALAM. Recovery of gold and silver from spent mobile phones by means of acidothiourea leaching followed by adsorption using biosorbent prepared from persimmon tannin, Hydrometallurgy. 133, 84-93, 2013. https://doi.org/10.1016/j.hydromet.2012.12.003.
D. ALVEAR, S. GALEAS, AND A. DEBUT. Síntesis y Caracterización de Nanopartículas de Magnetita, Rev. Politécnica, vol. 39, no. 2, pp. 61–66, 2017.
D. P. ROBLES ARDILA, N. RODRÍGUEZ PARDO, AND A. PATAQUIVA-MATEUS. Síntesis de nanopartículas de magnetita a partir del extracto de cáscara de papaya para la degradación de colorantes azoicos en soluciones acuosas, Rev. Chil. Ing., vol. 27, no. 3, pp. 431–442, 2019.
A. M. AWWAD AND N. M. SALEM. A Green and Facile Approach for Synthesis of Magnetite Nanoparticles, Nanosci. Nanotechnol., vol. 2, no. 6, pp. 208–213, 2013.
















