Autores
Adauto, A. (UNIVERSIDAD NACIONAL DE INGENIERIA) ; Morantes, G. (UNIVERSIDAD NACIONAL DE INGENIERIA) ; Fermin, B. (UNIVERSIDAD NACIONAL DE INGENIERIA) ; Picasso, G. (UNIVERSIDAD NACIONAL DE INGENIERIA)
Resumo
A computational study was carried out to select the monomer most related to the
drug IVER using the Gaussian 9.0 program with the semiempirical method. To
obtain the MIPs, IVER was used as the template molecule, 2-hydroxyethyl
methacrylate (HMA) or N-hydroxymethylacrylamide (HEMA) as functional monomer,
trimethylolpropane (TRIM) as crosslinking agent, 4,4′-azobis (4-cyanovaleric
acid) (ACVA) as radical initiator and acetonitrile as porogenic solvent. The
polymers were characterized using FTIR, TGA and N2 sorption. The maximum
adsorption capacities of IVER-MIP1 and IVER-NIP1 polymers in the removal of IVER
were 125,43 and 117,74 mg g-1, respectively. In all cases, the adsorption
kinetics and isotherm correlated best with the pseudo-second order and Langmuir
model, respectively.
Palavras chaves
Molecular imprinted polym; Ivermectin; Adsorption
Introdução
In the last three decades, emerging contaminants in aqueous systems have been
the subject of multiple research studies, the effects or damage to human health
and the environment are still unknown as there are no regulations governing the
presence of these new contaminants in the environment. However, many of these
pollutants could cause adverse effects to the ecological system and human
health, even at low concentration levels (NATARAJAN et al., 2021; NOGUERA-OVIEDO
& AGA., 2016). Pharmaceutical wastewater is part of these emerging pollutants
due to the presence of various types of drugs such as analgesics, antibiotics,
disinfectants and anticancer agents (SOPHIA & LIMA., 2018). The COVID-19 crisis
has raised great concern due to the harmful effects on the environment generated
not only by the massive use of masks and gloves but also by the drugs used to
treat it (WANG et al., 2022). Ivermectin is a macrocyclic lactone that exhibits
antiparasitic activity, antibacterial, antiviral and anticancer effects (Juarez
et al., 2018). Various methods have been used for the removal of
pharmaceuticals, with adsorption being one of the most efficient treatment
methods, due to its versatility, efficiency, ease of operation and low cost. The
MIPs perform selective adsorption due to their three-dimensional structure and
chemical composition. In addition, MIPs present numerous advantages, such as:
low cost, easy preparation, high mechanical and thermal resistance, they can be
reusable and stored for years without loss of efficiency. In terms of research,
few works oriented to the preparation of MIPs for ivermectin have been reported.
The aim of this work is the synthesis and characterization of MIPs and their
application in the removal of IVER.
Material e métodos
The optimization and energy values were obtained with the Gaussian 9.0
programmed using the semi-empirical method with AM1 and PM3. (COJOCARU et al.,
2013; KLEIN et al., 2006). The MIPs were synthesized using the precipitation
method, for which 0.05 mmol of the IVER was dissolved in 20 mL of acetonitrile
and 2.5 mmol of HMA (IVER-MIP1)or HEMA (IVER-MIP2), the mixture was stirred for
30 min at 350 rpm. Subsequently, 25 mmol of TRIM and 300 mg of ACVA were added
to the mixture and the solution was stirred. Afterwards, the mixture was purged
with N2 gas for 10 minutes. The polymerization was then carried out at 70°C for
12 hours. Finally, the polymeric materials were washed with a mixture of
methanol and acetic acid (9:1) for 72 hours to remove the IVER. The polymers
were washed with distilled water, dried at 60°C for 5 hours and sieved.
Regarding the synthesis of the NIPs, they were synthesized using the same
methodology as the MIPs, but in the absence of the template molecule. To
characterized the polymers, we used: infrared spectroscopy (FTIR) in the range
of 400-4000 cm-1, thermogravimetric analysis was performed in the range of 40° C
to 600° C and in N2 atmosphere and finally for the N2 sorption analysis, the
sample was subjected to a pre-treatment (degassing at 80°C for 2 hours). Kinetic
tests were performed in 2 mL vials, 2.5 mg of the synthesized materials were
weighed, then 2 mL of a 50 mg L-1 solution of IVER in 25% acetonitrile medium
was added. The mixture was stirred at 300 rpm at room temperature under various
time intervals: 5, 15, 30, 60, 120, 180, 240 and 300 min. Adsorption isotherm
tests were performed at room temperature by weighing 2.5 mg of the adsorbent
material and adding 2 mL of IVER solution at a concentration range of 50 to 500
mg L-1.
Resultado e discussão
The ivermectin molecule showed the strongest interaction with the HMA monomers
(-7.934 kcal mol-1), followed by the HEMA monomer (-7.895 kcal mol-1) and a very
weak interaction with the 4VP monomer (-0.249 kcal mol-1). Figure 1a shows a
band near 2985 cm-1 attributed to the C-H stretching, a band at 1720 cm-1
corresponding to the C=O bond of the HMA or HEMA and the TRIM and a band at 1620
cm-1 corresponding to the C=C stretching of the monomers (DU et al., 2018; DE
LIMA., 2016). In Figure 1b, 2 stages of mass decrease were observed, the first
stage was recorded at from 100 °C associated with the elimination of water and
solvent, and the second stage of decrease was recorded between 300-500 °C
related to degradation the polymeric chain (BAI et al., 2018), the latter
decrease was also observed in Figure 1c. The DTG curves show that the main
degradation peak is located between 400 and 500 °C. The isotherms shown in
Figure 1d present a behavior similar to that of type IV(a) with an H4-type
hysteresis loop (THOMMES et al., 2015). It was observed that MIPs presented
higher adsorption capacity compared to their respective NIPs, this would be due
to the imprinting process. Table 1 shows that the data were better adjusted to
the pseudo-second order model, obtaining a Qcal value similar to the Qexp value.
As for the Qe values: IVER-MIP1 (125.43 ± 0.08) > IVER-NIP1 (117.74 ± 0.15) >
IVER-MIP2 (106.31 ± 0.22)> IVER-NIP2(98.66 ± 0.07), this order is related to the
monomer used (HMA > HEMA). Table 2 shows an R2 value between 0.93 and 1 for the
Langmuir model, indicating that the adsorption of IVER on the polymers is a
monolayer adsorption where the active sites are energetically equivalent (ÖTER &
ZORER., 2021).
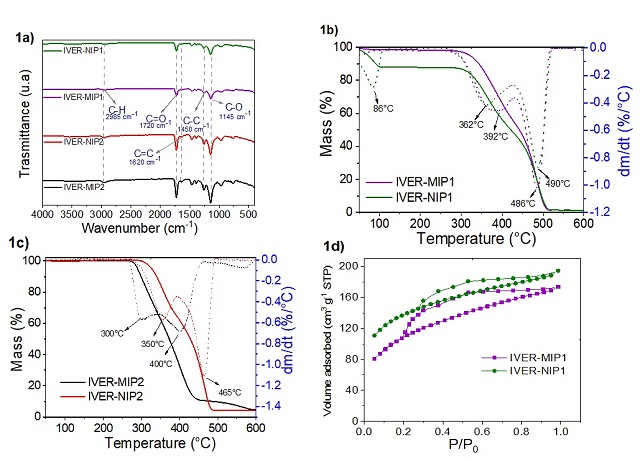
Figure 1. Characterization of polymers: 1a) Infrared spectra, 1b and 1c) TGA and DTG curves and 1d) N2 adsorption and desorption isotherms
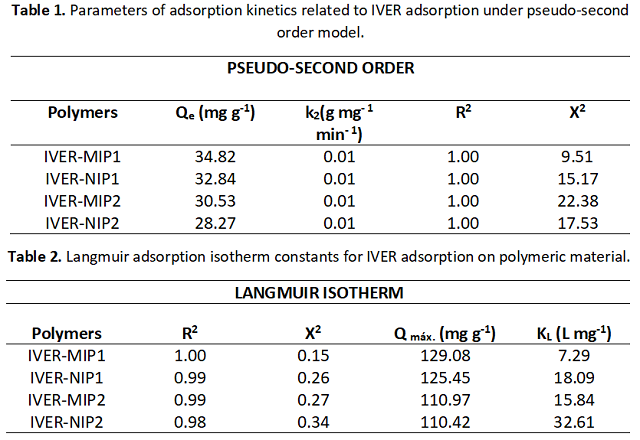
Table 2. Langmuir adsorption isotherm constants for IVER adsorption on polymeric material.
Conclusões
From the computational study, HMA and HEMA monomers were selected. The new
materials were obtained by a polymerization process. The monomers have atoms that
act as hydrogen bond donors and therefore form hydrogen bonds with the IVER drug
(O-H...O), generating high stability and selectivity in the polymer. The maximum
adsorption capacity was obtained with the IVER-MIP4 material (125 mg g-1). The
kinetic results and experimental isotherms were best fitted to the pseudo-second
order and Langmuir models, respectively.
Agradecimentos
To the programme of Special Projects: Projects for the Incorporation of
Postdoctoral Researchers in Peruvian Institutions (Agreement 057-2021-PROCIENCIA)
and the National University of Engineering of Lima-Peru.
Referências
BAI, J.; ZHANG, Y.; CHEN, L.; YAN, H.; ZHANG, C.; LIU, L.; XU, X. Synthesis and characterization of paclitaxel-imprinted microparticles for controlled release of an anticancer drug. Materials Science and Engineering: C, v. 92, n. 1, p. 338-348, 2018.
COJOCARU, C.; ROTARU, A.; HARABAGIU, V.; SACARESCU, L. Molecular structure and electronic properties of pyridylindolizine derivative containing phenyl and phenacyl groups: Comparison between semi-empirical calculations and experimental studies. Journal of Molecular Structure, v.1034, n. 1, p. 162-172, 2013.
DE LIMA, M.; VIEIRA, A.; MARTINS, I.; BORALLI, V.; BORGES, K.; FIGUEIREDO, E. On-line restricted access molecularly imprinted solid phase extraction of ivermectin in meat samples followed by HPLC-UV analysis. Food Chemistry, v. 197, n. 1, p. 7-13, 2016.
DU, W.; ZHANG, B.; GUO, P.; CHEN, G.; CHANG, C.; FU, Q. Facile preparation of magnetic molecularly imprinted polymers for the selective extraction and determination of dexamethasone in skincare cosmetics using HPLC. Journal of Separation Science, v. 41, n. 11, p. 2441–2452, 2018.
JUAREZ, M.; SCHCOLNIK-CABRERA, A.; DUENAS-GONZALEZ, A. Review the multitargeted drug ivermectin: from an antiparasitic agent to a repositioned cancer drug. American Journal of Cancer Research, v. 8, n. 2, p. 317–31, 2018.
KLEIN, E.; MATIS, M.; LUKEŠ, V.; CIBULKOVÁ, Z. The applicability of AM1 and PM3 semi-empirical methods for the study of N–H bond dissociation enthalpies and ionisation potentials of amine type antioxidants. Polymer Degradation and Stability, v. 91, n. 2, p. 262–270, 2006.
NATARAJAN, R.; BANERJEE, K.; KUMAR, P.; SOMANNA, T.; TANNANI, D.; ARVIND, V.; VAIDYANATHAN, V. Performance study on adsorptive removal of acetaminophen from wastewater using silica microspheres: Kinetic and isotherm studies. Chemosphere, v. 272, n. 1, p. 129896, 2021.
NOGUERA-OVIEDO, K.; AGA, D. Lessons learned from more than two decades of research on emerging contaminants in the environment. Journal of Hazardous Materials, v. 316, n. 1, p. 242–251, 2016.
ÖTER, Ç.; ZORER, Ö. Molecularly imprinted polymer synthesis and selective solid phase extraction applications for the detection of ziram, a dithiocarbamate fungicide. Chemical Engineering Journal Advances, v. 7, n. 1, p. 100118, 2021.
SOPHIA A., LIMA, E. Removal of emerging contaminants from the environment by adsorption. Ecotoxicology and Environmental Safety, v. 150, n. 1, p. 1–17, 2018.
THOMMES, M.; KANEKO, K.; NEIMARK, A.; OLIVIER, J; RODRIGUEZ, F.; ROUQUEROL, J.; SING, K. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure and Applied Chemistry, v. 87, n. 1, p. 9-10, 2015.
WANG, Q.; MIN, ZHANG.; RONGRONG, LIA. The COVID-19 pandemic reshapes the plastic pollution research – A comparative analysis of plastic pollution research before and during the pandemic. Environmental Research, v. 208, n.1, p. 112634, 2022.
















