Autores
Machado, I.B. (IFTO CAMPUS PARAÍSO DO TOCANTINS) ; Viroli, S.L.M. (IFTO CAMPUS PARAÍSO DO TOCANTINS) ; Costa, F.A. (IFTO CAMPUS PARAÍSO DO TOCANTINS) ; Veloso, C. (IFTO CAMPUS PARAÍSO DO TOCANTINS) ; Gomes, N.B. (IFTO CAMPUS PARAÍSO DO TOCANTINS)
Resumo
The teaching of chemistry contributes to the rational formation of high school
students, but it has been observed that many students show intense disinterest,
due to strictly expository classes and lack of experimental practice. The study
presents an approach to the processes of heat release and absorption through an
experimental practice to explain to students the inseparability between theory
and practice, since 2nd year high school students find it difficult to
understand the content. The experiment demonstrated effectiveness in explaining
the content about exothermic and endothermic reactions, providing a new view of
the processes that are presented to them in theory, establishing the importance
of experimentation in the learning process.
Palavras chaves
Thermochemistry; Chemistry teaching; Experimentation
Introdução
Chemistry teaching contributes a lot to the intellectual formation of elementary
and high school students, but many students show intense disinterest due to
strictly expository classes and lack of experimental practice (NETO et al,
2018). Experimental classes as a didactic approach in science teaching are of
great relevance for student learning, helping to understand chemical phenomena,
arouse interest among students, enable motivation and above all impress
students' senses (OLIVEIRA et al, 2018). Experimental classes enable learning
and analysis of the investigation of natural phenomena to solve problems
(GONÇALVES and GOI, 2020). Conducting the teaching of chemistry solely through
the lecture becomes monotonous, discouraging and hindering the assimilation of
contents. If the teaching given only with the use of experimental classes will
not be assimilated properly, as a theoretical basis is necessary to understand
the contents (NETO et al, 2018). An experiment associates motivation with
learning, as the participation of students in the construction of the chemistry
sample provides knowledge through discovery (SANTANA et al, 2019). Also
according to Santana et al. (2019) this is the time to search for setting goals
and ways of implementing the proposals established, provoking curiosity,
criticality and competitiveness for knowledge, thus corroborating for the more
consistent development of learning. Given the above, the objective of this study
is to present an approach to the processes of heat release and absorption
studied in Thermochemistry through an experimental practice to explain to
students the inseparability between theory and experimentation, since 2nd year
students High School students see the content as difficult to understand and
assimilate.
Material e métodos
The study carried out was of an experimental quantitative investigative nature,
where 24 students from the 2nd year of High School Integrated to the
Agroindustry Technician course of the Federal Institute of Education Science and
Technology of Tocantins: campus Paraíso do Tocantins using a script to determine
the amount of heat involved in chemical transformations through exothermic and
endothermic reactions, carried out two experiments on the content of
Thermochemistry. In the first experiment: by frying an egg through a chemical
reaction, 10 g of sodium hydroxide (NaOH) were weighed using a watch glass on an
analytical balance, after weighing NaOH was dissolved in 50 ml of water in a
beaker. The NaOH solution was covered with aluminum foil inside the beaker,
white and egg yolk were added to the aluminum foil and the process of releasing
the heat from the reaction of the aluminum foil with the NaOH solution was
allowed to “fry” the egg. In the second experiment: freezing the water through a
chemical reaction, the students separately weighed 11 g of ammonium chloride
(NH4Cl) and 32 g of barium hydroxide (Ba(OH)2) using a
watch glass on an analytical balance. Add the NH4Cl and Ba(OH)2 in a
flat-bottomed flask, using a funnel and spatula with stirring until a liquid
solution forms. Then, the flask was placed in a large Petri dish with water
where it was possible to observe the freezing of the water. After carrying out
the experiments, the students answered a questionnaire with the following
questions: Did the experimentation contribute to the learning about the concepts
of absorption and heat release in chemical reactions? Was learning through
experiments motivating? The experimental class made it possible to understand
thermochemistry.
Resultado e discussão
In the first experiment, it was possible to demonstrate the increase in
temperature caused by the release of heat (ANTUNES, 2013), an exothermic
reaction, caused by the reaction of aluminum foil and the NaOH solution to the
point of providing enough thermal energy to “fry” the egg, as shown in Figure 1.
In the second experiment, the concept of endothermic reaction was verified, that
is, a reaction that takes place with heat absorption (NETO et al, 2018). When
Ba(OH)₂ and NH₄Cℓ are stirred, they form a liquid solution, causing a decrease
in temperature, causing the system to cool and freeze the water contained in the
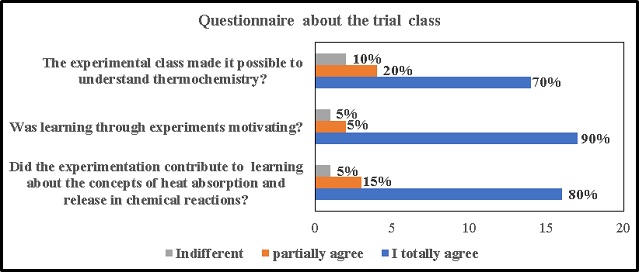
Petri dish according to Figure 2. According to the results obtained in the
questionnaire and shown in graph 1, we observed that 70% of the students fully
agreed that the experimental class allowed for an understanding of
thermochemistry, favoring the concepts of exothermic and endothermic reaction.
When asked about learning through experiments was motivating, 90% strongly
agreed and when asked about the contribution of experimentation to learning
about the concepts of absorption and heat release in chemical reactions, 80%
strongly agreed that it contributes. Santana et al, (2019). state that
experiments provide students with an opportunity to appropriate scientific
concepts through investigation, seeking to understand the transformations that
occurred during experimentation. Francisco Jr., Ferreira e Hertwig (2008) say
that the experimental approach of an investigative nature aims to obtain
information to support the discussion, reflection, considerations and
explanations, leading the student to understand the concepts and different way
of thinking about the world through science.

Conclusões
The realization of the experimental class reached the desired objective,
verifying the heat involved in chemical transformations through exothermic and
endothermic reactions in everyday life, indicating the inseparability of theory
and practice, allowing students to experience to build their knowledge without
memorizing formulas. The experiment demonstrated effectiveness in explaining the
content about exothermic and endothermic reactions, providing a new view of the
processes that are presented to them in theory, establishing the importance of
experimentation in the learning process.
Agradecimentos
TO GOD, to the IFTO Paraíso do Tocantins campus
Referências
ANTUNES, M.T. Ser protagonista: Química Ensino Médio 3ºano. 2.ed. São Paulo: Edições SM., 2013.
FRANCISCO JR, W. E.; FERREIRA, L. H.; HARTWIG, D. R. Experimentação Problematizadora: Fundamentos Teóricos e Práticos para a Aplicação em Salas de Aula de Ciências. Química Nova. Disponível em:< http://qnesc.sbq.org.br/online/qnesc30/07-PEQ-4708.pdf> Acesso em 20 abr. 2022.
GONÇALVES, R. P. N.; GOI, M. E. J.. Experimentação no Ensino de Química na Educação Básica: Uma Revisão de Literatura. Revista Debates em Ensino de Química - Redequim, v. 6 n. 1. 2020.
NETO, D.R.N.; SANTOS, G.L.S.; CARVALHO, J.A.; ARAÚJO, H.F. A experimentação como uma proposta didática no ensino de termoquímica com ênfase nos processos exotérmicos e endotérmicos. In: CONGRESSO BRASILEIRO DE QUIMICA, 58., 2018, São Luis. Anais Eletrônicos [...]. São Luis, 2018. Disponível em: http://www.abq.org.br/cbq/2018/trabalhos/6/1853-23421.html. Acesso em: 25 maio 2022
OLIVEIRA, L.C.S.; MARES, E.K.L.; MELO, K.C.; SOUZA, D.A.A.; CAMPOS, W.E.O.; CONCEIÇÃO, L.R.V. A experimentação na produção e crescimento de cristais de sais duplos. In: CONGRESSO BRASILEIRO DE QUIMICA, 58., 2018, São Luis. Anais Eletrônicos [...]. São Luis, 2018. Disponível em: http://www.abq.org.br/cbq/2018/trabalhos/
6/1982-23566.html. Acesso em: 25 maio 2022
SANTANA, R.O.; SILVA, R.C.; VIANA, C.C.; JOSAPHAT, E.; RAMOS, L.P.; CHERMONT, J.N.M.; QUEIROZ, F.A.; SANTOS, L.J.S. A experimentação como ferramenta pedagógica na amostra de química: socializando saberes com produções de estudantes do ensino médio. In: CONGRESSO BRASILEIRO DE QUIMICA, 59., 2019, João Pessoa. Anais Eletrônicos [...]. João Pessoa, 2019. Disponível em: http://www.abq.org.br/cbq/2019/trabalhos/6/370-26391.html. Acesso em: 25 maio 2022
















