Autores
Carvalho, N.P. (IFTO CAMPUS PARAÍSO DO TOCANTINS) ; Viroli, S.L.M. (IFTO CAMPUS PARAÍSO DO TOCANTINS) ; Alves, T.T. (UFT CAMPUS GURUPI) ; Teles, T.N. (IFTO CAMPUS PARAÍSO DO TOCANTINS) ; Lança, A.C. (UFT CAMPUS GURUPI)
Resumo
Chemistry teaching needs classes that involve students with investigative
activities. The objective of the study was the construction of an alternative
calorimeter with low-cost material. The study was carried out with 25 students
from the 2nd year of high school at a public school. The constructed calorimeter
was used to determine the enthalpy of neutralization. After the experiment the
students answered a questionnaire the experiment. The experiment was of great
pedagogical importance, as it contributed to the development of significant
knowledge aimed at improving teaching.
Palavras chaves
Investigative teaching; neutralization reaction; experimentation
Introdução
The lack of perception of most students in relating Chemistry with various
processes present in everyday life occurs most of the time because they do not
have a contextualized learning during classes (DIONIZIO, 2018). Chemistry
teaching must go beyond the memorization of formulas, disconnection between
practice and theory, and classes that allow investigative activities must be
applied (SASSERON 2013). Investigative teaching encourages the student to
understand natural phenomena by establishing a connection with scientific
knowledge (CARVALHO, 2013). Practical classes enable students to learn in the
conception of concepts and motivation in the analysis of the investigation of
natural phenomena to solve problems (GONÇALVES and GOI, 2018). Experimentation
is a widely discussed and defended pedagogical resource in chemistry teaching,
whose intention is to arouse motivation, interest and analysis of the
theoretical knowledge associated with the experiment (GREIN, 2014; OLIVEIRA et
al., 2014). According to Henzel (2019) most Brazilian public schools do not have
adequate infrastructure, materials or laboratories that allow the execution of
the experimental activity, with the need to search for alternatives that make it
possible to carry out the class, experiments such as: non-formal spaces,
material Low cost, recycled materials. Therefore, the objective of this study is
the construction of an alternative calorimeter with low cost material, being a
facilitator instrument in chemistry classes, helping in the learning of students
of the 2nd year of high school in a public school, from the experimental
results. obtained as a complementary activity to lectures on the content of
thermochemistry, as well as awakening in the student an investigative, critical
and motivating vision.
Material e métodos
The study was carried out in a state public school, located in the Setor Oeste
neighborhood in the city of Paraíso do Tocantins. Twenty-five high school 2nd
year students participated in the research. A study on calorimetry and enthalpy
of neutralization was carried out. These chemical contents are included in the
Physical Chemistry subject taught in the 2nd year of High School. After the
study, a survey was carried out on what materials would be needed for the
construction of the equipment. The students were divided into 5 groups. Each
group received the following materials: styrofoam packaging, wire, soda can,
detergent lid and thermometer to build the calorimeter. To build the
calorimeter, the students initially removed the top of the soda can with a can
opener. They cut 35 cm of wire and twisted one end to cause agitation when it
was inside the liquid in the calorimeter. They divided the styrofoam packaging
in half, with one half having two holes drilled. In one of the holes, the
detergent cap was added to introduce the thermometer and the other hole was the
wire to provoke the agitation of the liquid in the calorimeter. The soda can was
placed inside one half of the styrofoam package and covered with the other half.
Figure 1 shows the procedures used in the construction of the calorimeter.The
constructed calorimeter was used in an experiment to determine the enthalpy of
neutralization between strong acidic and basic solutions. After the experiment,
an evaluative questionnaire was prepared on the construction and use of the
calorimeter in the experiment carried out, which students would have to answer
about learning and participation in the experience.
Resultado e discussão
There was intense student participation during the calorimeter construction
stages and the experimental class. They were very receptive to the knowledge
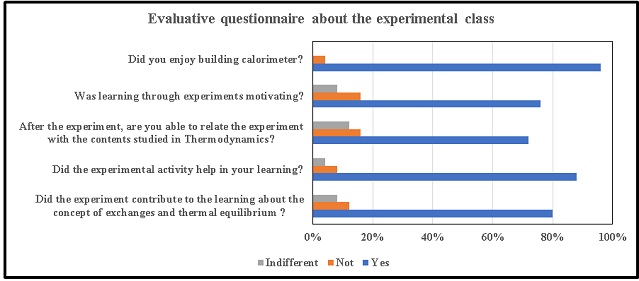
covered, taking measurements and handling the equipment. Graph 1 shows the
results of the questionnaire after the construction and use of the calorimeter
used in the experimental class. According to the graph: 80% of the students
agreed that the experiment helped to learn about the concept of exchanges and
thermal equilibrium and to determine the heat of naturalization between an acid
and a base, with 12% disagreeing and 8% indifferent. 88% stated that the
experimental activity helped in learning, 8% disagreed and 4% were indifferent.
When asked about the ability to relate the experiment with the contents studied
in Thermodynamics, 72% answered yes, 16% answered no and 12% were indifferent.
76% said learning through experimentation was motivating, 16% said it wasn't,
and 8% were indifferent. With regard to liking having participated in the
construction of the calorimeter, 96% said yes and 4% did not like having
participated. According to Pereira (2019), carrying out an experiment with the
construction and use of a calorimeter found an increase in student interest and
learning. Oliveira et al. (2019), applying a questionnaire on the use of a
calorimeter in an experimental class, found that after carrying out the
practice, 92% agreed with this new teaching methodology and 8% did not. Also
according to the authors, the great lack of interest of students in the study of
chemistry is due, in general, to the lack of experimental activities that can
relate theory and practice. According to Zanon and Uhmann (2012) experimentation
helps students in the investigative process in order to signify such concepts.

Conclusões
The difficulties experienced in some public schools in the teaching of Chemistry
demonstrate the need for innovative practices, through didactic tools capable of
arousing the interest of students. The experiment carried out was of great
pedagogical importance, exploring various chemical contents. The construction of
an alternative calorimeter made with low-cost material aided learning,
collaborating with the development of significant knowledge aimed at improving
teaching through better absorption of knowledge about the study of enthalpy of
neutralization.
Agradecimentos
TO GOD, to the IFTO Paraíso do Tocantins campus
Referências
CARVALHO, A. M. P. de. O Ensino de Ciências e a proposição de sequências de Ensino Investigativas. In: CARVALHO A. M. P. de. (Org.). Ensino de Ciências por investigação: condições para implementação em sala de aula. 1ed. São Paulo: Cengage Learning, v. 1, pp. 1 - 20, 2013
DIONÍZIO, T. P. “Uno da Química”: conhecendo os elementos químicos por meio de um jogo de cartas. Revista Educação Pública, Rio de Janeiro, v. 18, nº 14. 2018. DOI: 10.18264/REP Disponível em: https://educacaopublica.cecierj.edu.br/artigos/18/14/ldquo-uno-da-qumica-rdquo-conhecendo-os-elementos-qumicos-por-meio-de-um-jogo-de-cartas. Acesso em: 12 abr 2022.
GONÇALVES, R. P. N.; GOI, M. E. J. Experimentação no Ensino de Química na Educação Básica: Uma Revisão de Literatura. Revista Debates em Ensino de Química - Redequim, v. 6 n. 1. 2020.
GREIN, A. C. V. Desenvolvimento de senso crítico, analítico e científico em alunos participantes de clube de ciências. 2014. 79 f. Dissertação (Mestrado em Ciências) - Universidade Tecnológica Federal do Paraná, Programa de Pós-Gradução em Formação Científica, Educacional e Tecnológica, Curitiba, 2014.
HENZEL, T. L. A utilização da experimentação na sala de aula. Revista Insignare Scientia - RIS, v. 2, n. 3, p. 323-330. DOI: https://doi.org/10.36661/2595- 4520.2019v2i3.11214 nov. 2019. Disponível em: https://periodicos.uffs.edu.br/index.php/RIS/article/view/11214. Acesso em: 12 abr 2022.
OLIVEIRA, R. V.; PIMENTA, D. B.; SILVA, M. R. S. L da.; DORNELES, E., P. Utilização de Materiais Caseiros para Experimentação em Laboratório no Ensino de Química, V Encontro Nacional das Licenciaturas, UFRN, Anais do V ENALIC, Natal-RN, 2014.
OLIVEIRA, L.; SIQUEIRA, L.; SANTOS, J.; OLIVEIRA, N. Construção de calorímetro com material alternativo para auxiliar o aprendizado de termoquímica. In: CONGRESSO BRASILEIRO DE QUIMICA, 59., 2019, João Pessoa. Anais Eletrônicos [...]. João Pessoa, 2018. Disponível em: https://www.abq.org.br/cbq/2019/trabalhos/6/1798-28284.html. Acesso em: 25 maio 2022
PEREIRA, F. G. Proposta e análise de uma sequência didática para abordar o conteúdo de termoquímica no ensino médio. Dissertação (Programa de Pós-Graduação em Ensino de Ciências e Matemática) - Universidade Federal de Uberlândia. Minas Gerais, 118p. 2019.
SASSERON, L. H. Interações discursivas e investigação em sala de aula: o papel do professor. In: Anna Maria Pessoa de Carvalho. (Org.). Ensino de Ciências por investigação: condições para implementação em sala de aula. 1ed.São Paulo: Cengage Learning, v. 1, pp. 41 - 62, 2013.
ZANON, L. B.; UHMANN, R. I. M. O desafio de inserir a experimentação no ensino de ciências e entender a sua função pedagógica. In: ENCONTRO NACIONAL DE ENSINO DE QUÍMICA, 16., ENCONTRO DE EDUCAÇÃO QUÍMICA DA BAHIA, 10., Salvador. Anais... Salvador: UFBA, 2012.
















