Autores
Mayta, S. (UNIVERSIDAD NACIONAL DE INGENIERIA) ; Huamani-palomino,, R.G. (UNIVERSIDAD NACIONAL DE INGENIERIA) ; Córdova, B.M. (UNIVERSIDAD NACIONAL DE INGENIERIA) ; Marín, N. (UNIVERSIDAD NACIONAL DE INGENIERIA) ; Quintana, M. (UNIVERSIDAD NACIONAL DE INGENIERIA) ; Rivera, E. (UNIVERSIDAD NACIONAL AUTÓNOMA DE MÉXICO)
Resumo
Corn husk is one of the most abundant agricultural waste worlwide. This biomass
constitutes a cellulose-rich byproduct which its valorization is still a
challenge. In this study, corn husk cellulose was obtained by different methods,
including the study of an Organosolv pretreatment and different bleaching agents
(H2O2, NaClO2, and peracetic acid).It was found that the NaClO2-bleached cellulose
sample had the highest crystallinity index (45.6), at the same time peracetic
acid-bleached cellulose had the highest purity and brightness compared to the ones
obtained by NaClO2 and H2O2 bleaching, while the greatest yield was obtained with
NaClO2(74% weight).This work provides a new approach for lignocellulosic biomass
bleaching with peracetic acid, which is a effective and biodegradable compound
Palavras chaves
CORN HUSK WASTE; cellulose; bleaching
Introdução
Out of all the cereals, maize has the highest production volume with more than 1
billion tons per year (Faostat,2003).
In Peru, according to data from the Minister of Agriculture (MIDAGRI, 2021),
corn is the most harvested cereal which is mainly grown in the coastal valleys
of Lima and Ica regions. Regarding the level of production, during 2020 a
production of 414 thousand tons were reported approximately, leaving
considerable amounts of wastes (nearly 50%) after its commercialization.
At the same time, it is estimated that cereals are the ones that leave the most
amount of residues on the soil surface and are also considered a feedstock that
is very difficult to degrade.
In particular, out of the total maize plant only 50% corresponds to grain, the
other 50% is made up of leaves, husk, canes and cobs ( Huamanchumo, 2013).
In that sense, corn husks constitute a significant portion of the total waste
and in most cases it's mainly disposed of by open-burning generating significant
amounts of greenhouse gasses and particulate matter, which has a considerable
impact on ecosystems, in addition to the risk of fire that it implies.(Carrasco
& Aguirre, 2018)
On the other hand, scientists have shown a particular interest on valorization
of different lignocellulosic biomass over the last decade, as it constitutes the
largest source of renewable organic material on Earth (Mussato, 2016).
This type of biomass includes, among others, agricultural / agro-industrial
residues (sugar cane bagasse, corn stover, corn cob, rice husks, rice straw,
wheat straw, used grains, among others) (Clauser, 2019), and woody materials
(wooden branches, bark, and logs, as well as wood waste from sawmills,
packaging, and wooden pallets) (Limayem & Ricke, 2012).
Lignocellulosic biomass mainly consists of a mixture of polymers based on
lignin, cellulose and hemicellulose.
In the case of corn husk wastes -compared to different ago wastes sources-few
researches have been conducted aiming to valorize its constituents According to
(Smyth et al, 2017) corn husk are composed of cellulose, lignin and
hemicellulose at 31.3%,48.9% and 10.9% in weight, respectively.
Indeed, the fractionation of biomass consists of applying different treatments
to separate each of the components of the lignocellulosic materials so that they
can be processed more easily and subsequently functionalized. In addition, the
type of treatment to apply, and the sequence of these, depends on the component
up to extraction, whether it would be cellulose, hemicellulose (Arzami et al,
2022) and/or lignin (Cassoni et al, 2022) . However, the recalcitrant nature of
these materials (resistance of the plant cell wall to its deconstruction) makes
its valorization a challenge (Mussato, 2016)
According to (Clauser, 2019), the integral processing of lignocellulosic biomass
includes: mechanical operations (for example: pressing, grinding , size
reduction, extrusion( Hietala et al, 2013), thermochemical (for example:
combustion (Carmona, V.B. et al., 2013), pyrolysis, liquefaction), biochemical
(for example: fermentation, enzymatic processes (Squinca, P. et al., 2022) and
chemical (for example: electrolysis of water, hydrogenation, esterification,
oxidation).
In that sense, cellulose is the main component of lignocellulosic biomass, it is
a linear homopolymer of glucose units (C6H12O6) linked together in the form of
D-anhydro glucopyranose units through glycosidic bonds. Typically, each
cellulose molecule comprises between 5,000 and 10,000 glucose units (degree of
polymerization). At the structural level, intra- and intermolecular hydrogen
bridge bonds lead to the formation of a rigid network made up of cellulose
fibers. Also, regarding the cellulose structure we distinguish between
crystalline (highly ordered) and amorphous (less ordered) regions, the
crystalline regions are more difficult to hydrolyze (enzymatically or
chemically) to molecules of lower molecular weight compared to the amorphous
regions (SUN, 2010 ).
A critical step when extracting cellulose is bleaching. It provides cellulose
with greater brightness and higher purity. Chlorine and hypochlorite compounds
have been widely used for cellulose bleaching due to their low cost and high
effectiveness. (SHARMA ET AL, 2020). However these compounds are responsible for
major impacts in the environment, due to the formation of toxic and non-
biodegradable derivatives.
Peracetic acid (PAA) or Peroxyacetic acid, is a strong oxidant, colorless,
biodegradable and corrosive organic chemical. Its Oxidation potential is higher
than chlorine or chlorine dioxide but less than ozone (SHARMA ET AL, 2020).
In that sense, peracetic acid seems a promising substitute to replace the
chlorine bleaching and has been widely used in th used in the cotton industry.
(ABDEL-HALIM & AL-DEYAB, 2011)
Under that context, this research focuses on the evaluation of different
bleaching agents for cellulose extraction from corn husk agro waste.
Material e métodos
All reagents were analytical grade and were used without any modification.
During all operations, deionized water with a pH of 7 and a conductivity of 3 µS
was used.
Corn husks were collected from local markets in the city of Lima, Peru.
Subsequently, they were classified and washed twice.
The previously washed and classified maize husks were placed in an oven at 45°C
until there was no change in the mass.
Cellulose Extraction:
Two extraction methods were implemented. The first one consisted of the
following operations: Organosolv Pretreatment, Alkaline Treatment and Bleaching,
while in the second method the Organosolv pretreatment was omitted, maintaining
the same sequence and incorporating three different bleaching agents: sodium
chlorite, hydrogen peroxide and peracetic acid.
It is important to note that this is the very first research that incorporates
peracetic acid as a bleaching agent for cellulose obtained from corn agro waste.
Organosolv Pretreatment (OP)
The Organosolv pretreatment was carried out in a 5 L SS-316 High Pressure
Autoclave-Reactor The reactor was loaded with 120 g of dried corn husk chips and
1.6 L of a 50% (v/v) ethanol-water solution.The reaction mixture was stirred at
250 rpm and at the set temperature (95 C). The mixture was vacuum filtered and
the solid was washed.
Alkaline Treatment (AT)
The corn husk -previously conditioned- was treated with a 4% w/v sodium
hydroxide solution at 80°C for 4 hours. The solid was then filtered and washed
several times with distilled water until the filtrate had a neutral pH. The
treatment was repeated twice. The pulp resulting from this treatment was stored
in a container and immediately disposed of in subsequent treatments.
Bleaching Treatment (BT)
After alkaline treatment, bleaching was carried out using three different
reagents: sodium chlorite (NaClO2), hydrogen peroxide (H2O2) and peracetic acid
(PAA).
When bleaching with sodium chlorite, a 1.7% w/v solution was used, then the
solution was acidified to a pH of 3.8. The mixture was placed in a 1L flask and
boiled in reflux equipment under the action of an air extractor hood.
Subsequently, the mixture was allowed to cool and the solid was simultaneously
washed and vacuum filtered with distilled water.
Similarly, when bleaching with hydrogen peroxide.This mixture was allowed to
react for 2 hours and at 60°C.Subsequently, the mixture was simultaneously the
solid was washed and vacuum filter.
Finally, for the study of bleaching with peracetic acid (PAA),
The PAA was prepared in situ: 0.5 mol of glacial acetic acid was mixed with
drops of sulfuric acid, after cooling, 0.5 mol of hydrogen peroxide was added to
form a total volume of 100mL. The pH was then adjusted to 7 and the pulp from
the PA was immediately poured into the solution. This mixture was treated at
60°C for 1 hour. Lastly, the solid was washed and vacuum filtered with deionized
water until the liquor had a neutral pH.
Resultado e discussão
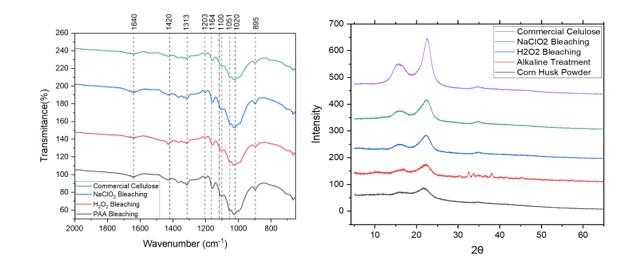
The X-ray diffractograms of commercial cellulose, corn husk powder and two
different bleached cellulose samples are shown in fig. 1.
The main intense peaks in the diffractogram are located at 2θ value of around
17◦, 22◦. These peaks correspond to lattice planes (110) and (200),
respectively. (RIZWAN ET AL 2021)
At the same time sharp peaks are indicative of the crystalline nature of
cellulose (unlike hemicellulose and lignin). It is important to note that
sharper peaks are observed as we move forward in the treatments( untreated
sample, alkaline treatment, bleaching), this confirms the release of crystalline
domains due to the effectiveness of the treatment.
The crystallinity index for cellulose can be calculated by using the Segal
equation.
The CI of the NaClO2 is calculated as 45.6 which is higher than the 43.2 CI of
the H2O2 bleached cellulose but lower than the 57 CI of the commercial
cellulose.
A higher CI of the extracted cellulose corresponds to a greater removal of the
amorphous hemicellulose and lignin by both alkaline and bleaching treatments.
(NIGAM ET Al, 2021)
The cellulose obtained by NaClO2 bleaching had a 72% of the CI value of
cellulose obtained from eucalyptus and 79% of the CI value of cellulose obtained
from bagasse, two typical woody and non woody sources. (WEI ET AL, 2017)
(remarcar mejores resutlados para el blanqueado del paracetico)
FT-IR analysis was conducted to investigate the presence of different functional
groups among the spectral bands. Figure 1. shows the IR spectra of different
bleached samples and commercial cellulose.
Two broad peaks in the region of 2900-3500 cm− 1(not shown in the spectra) are
characteristic of cellulose. The former at 3300 correspond to the O-H stretching
vibration while the latter at 2900 correspond to the C-H stretching vibration.
(RIZWAN ET AL 2021)
At 1640 cm-1 we recognize signals of the C=O stretching vibrations , however
signals of O-H bending of the absorbed water are also present in the region,
this goes in agreement with the tendency of lignocellulosic biomass to retain
water (MUSSATO, 2016), so in order to overcome this effect an effective drying
technique must be applied.
Around 1412 cm-1 we find a signal attributed to the C=H deformation in methyl
groups of lignin rings.(NIGAM ET Al, 2021). This band is relatively more intense
in the H2O2 bleached cellulose , which also corresponds to the fact that this
cellulose has a light brown coloration , typically from the presence of
remaining lignin.
At 1313-1327 cm-1 we find signals corresponding to C-C and C-O skeletal
vibrations (KHENBLOUCHE ET AL, 2019).The absorption band at 1118 cm−1 was
attributed to C-OH skeletal vibration, while the C-O-C pyranose ring skeletal
vibration showed a band at 1048 cm−1(KALLEL ET AL ,2016).
The sharp band at 895 cm− 1 is attributed to the presence of characteristic C-O-
C stretching β-glycosidic linkages between the sugar residues(NIGAM ET AL,
2021).
In all cases, typical peaks of lignin and hemicelluloses were not present,
indicating the removal of these two components. These signals are usually shown
at 1750 cm-1 (C=O stretching in acetyl groups) in hemicelluloses, and at 1520
cm−1- 1250 cm−1 for lignin (assigned to C=C and C=O stretching vibrations).
Finally, more intense peaks at certain wavenumbers indicate larger amounts of
pure cellulose (SMYTH ET AL,2017). In this case the growth of intensity of peaks
occurred in the next order: PAA bleached cellulose > NaClO2 bleached cellulose >
H2O2 bleached cellulose.
Regarding the SEM images, we can distinguish the changes along the structure of
the material, in the very first cases the corn feedstock has an irregular and
rough surface due to the content of not only structural components but also the
extractives. On the latter images (Organosolv-isolated cellulose) it is very
clear the fibrillation in the structure. These cellulose microfibers indicate
that both lignin and hemicellulose, which both act as linkers of these fibers,
have been removed, releasing the crystalline-cellulose fibers.
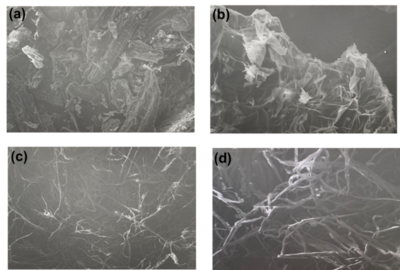
Scanning electron micrographs of untreated corn husk powder (CHP) (a) at 150X and (b) at 500X resolutions; scanning electron micrographs of cellulose
Conclusões
Cellulose was successfully isolated from corn husk under different procedures,
with an outsatding peracetic acid bleaching performance in brightness and purity,
this biodegradable compound does not release harmfull efluents -like chlorine
agents- and yet showed strong oxidation properties.
It was also remarkable the effect of organosolv pretreatment in the fibrillation
of cellulose, which also had an effective role in the delignification process
removing both lignin and hemicellulose. Finally it was shown that corn wastes
constitute a promising source for high purity cellulose isolation and its large
scale production still needs atention, even more considering the huge volumens of
this waste.
Agradecimentos
This work was financially supported by PROCIENCIA through the project N°204-
FONDECYT-2020.
Referências
ARZAMI, A.N., HO, T.M. & MIKKONEN, K.S., 2022. Valorization of cereal by-product hemicelluloses: Fractionation and purity considerations. Food Research International, 151, p.110818.
CARMONA, V.B. ET AL., 2013. NANOSILICA FROM RICE HUSK: Extraction and characterization. Industrial Crops and Products, 43, pp.291–296.
CASSONI, A.C. ET AL., 2022. Systematic Review on lignin valorization in the agro-food system: From sources to applications. Journal of Environmental Management, 317, p.115258.
CARRASCO, J., Y AGUIRRE, C. (EDS.) 2018. Rastrojos del cultivo de Maíz: elementos a considerar para su manejo. Boletín INIA Nº 385. Instituto de Investigaciones Agropecuarias, Centro Regional Rayentué. Rengo, Chile.
CLAUSER, N., 2019. Estudio técnico-económico de la biorrefinería de los residuos de industrialización primaria de la madera y agroindustriales Universidad Nacional de Misiones. Facultad de Ciencias Exactas, Químicas y Naturales.Argentina
HIETALA, M., MATHEW, A.P. & OKSMAN, K., 2013. Bionanocomposites of thermoplastic starch and cellulose nanofibers manufactured using twin-screw extrusion. European Polymer Journal, 49(4), pp.950–956.
FAO STADISTICS 2013, Food and agricultural organization of the United Nations.
HUAMANCHUMO DE LA CUBA, C & INSTITUTO INTERAMERICANO DE COOPERACIÓN PARA LA AGRICULTURA (IICA), (2013). La cadena de valor de maíz en el Perú: diagnóstico del estado actual, tendencias y perspectivas
KALLEL, F. ET AL., 2016. Isolation and structural characterization of cellulose nanocrystals extracted from garlic straw residues. Industrial Crops and Products, 87, pp.287–296.
KHENBLOUCHE, A. ET AL., 2019. Extraction and characterization of cellulose microfibers from Retama raetam stems. Polímeros, 29(1).
LIMAYEM, A., & RICKE, S. C. (2012). Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues, and future prospects. Progress in Energy and Combustion Science, 38(4), 449–467. https://doi.org/10.1016/j.pecs.2012.03.002
Ministerio de Desarrollo Agrario y Riego, Maíz Choclo, Semana Nacional de Frutas y Verduras 2021, (2020). Recuperado de https://cdn.www.gob.pe/uploads/document/file/1828781/Brochure_Mai%CC%81z%20Choclo.pdf.pdf?fbclid=IwAR1tiwUc6hZFhlxNTTtDak2v8CWU-c3SHr0oeDlz-Hi-4E67HHHoV--4GLI
MUSSATO S.I., Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery. (2016). Elsevier Science Publishing
REYES-MURO, L.; CAMACHO-VILLA, T. C.; GUEVARA-HERNÁNDEZ, F. (2013). Rastrojos: manejo, uso y mercado en el centro y sur de México. Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. Pabellón de Arteaga, Aguascalientes, México.
RIZWAN, M. ET AL., 2021. Cellulose extraction of alstonia scholaris: A comparative study on efficiency of different bleaching reagents for its isolation and characterization. International Journal of Biological Macromolecules, 191, pp.964–972.
SHARMA, N., BHARDWAJ, N.K. & SINGH, R.B., 2020. Environmental issues of pulp bleaching and prospects of peracetic acid pulp bleaching: A Review. Journal of Cleaner Production, 256, p.120338.
SMYTH, M., GARCÍA, A., RADER, C., FOSTER, E., & BRAS, J. (2022). Extraction and process analysis of high aspect ratio cellulose nanocrystals from corn (Zea mays) agricultural residue. Industrial Crops & Products 108 (2017) 257–266 http://dx.doi.org/10.1016/j.indcrop.2017.06.006
SQUINCA, P. ET AL., 2022. The use of enzymes to isolate cellulose nanomaterials: A systematic map review. Carbohydrate Polymer Technologies and Applications, 3, p.100212.
SUN, R. (2010). Cereal straw as a resource for sustainable biomaterials and biofuels: Chemistry, extractives, lignins, hemicelluloses and cellulose. Elsevier Science.
Wei, W.Q. & Wu, S.B., 2016. Conversion of eucalyptus cellulose into 5-hydroxymethylfurfural using lewis acid catalyst in biphasic solvent system. Waste and Biomass Valorization, 8(4), pp.1303–1311.
















