Autores
Meneses, L. (PONTIFICIA UNIVERSIDAD CATÓLICA DEL ECUADOR) ; Reinoso, C. (PONTIFICIA UNIVERSIDAD CATÓLICA DEL ECUADOR) ; Morales, F. (PONTIFICIA UNIVERSIDAD CATÓLICA DEL ECUADOR) ; Rengifo, M.J. (PONTIFICIA UNIVERSIDAD CATÓLICA DEL ECUADOR) ; Proaño-bolaños, C. (IKIAM)
Resumo
The rapid increase in microbial resistance presents a serious challenge for
antimicrobial therapies. The inefficiency of potent antibiotics against superbugs
makes the development of other control agents urgent. Within these new control
agents are Antimicrobial and antifungal peptides. In this work, the interaction of
dermaseptine and cruzioseptine peptides extracted from the skin of the Hylidae
family frog, that present antimicrobial activity were modeled computationally,
through methods of molecular coupling (Docking), in order to establish a mechanism
of action of these peptides against Escherichia coli, Staphylococcus aureus and
Candida albicans. The most likely mechanism of action would be the rupture of the
cell membrane by the action of these basic peptides.
Palavras chaves
peptides; antimicrobial activity; frogs
Introdução
The progressive increase in the use of antibiotics has led to antimicrobial
resistance, which has become a global problem, as it has been estimated that by
2050 it will be the leading cause of death in the world. The design of
antimicrobials that are not prone to the different mechanisms of resistance that
currently exist towards antibiotics by various microorganisms is quite
complicated since bacteria have three types of antimicrobial resistance.
Therefore, small-molecule antimicrobials such as antimicrobial peptides (AMPs)
are an option to replace conventional antibiotics.
AMPs are small molecules composed of 5-40 amino acids generated in different
organisms. AMPs are amphipathic and cationic, where their structure is composed
of hydrophobic and hydrophilic elements, with a net positive charge (in the
range of +2 and +9). These molecules can be effective against a wide variety of
microorganisms that are resistant to antibiotics.
There are some mechanisms of action of cationic AMPs. However, generally, these
peptides exhibit membrane-binding activity through interaction between positive
charges and negative charges, causing the formation of a cavity in cell
membranes, thus resulting in membrane permeability and eventually causing
overflow of bacterial contents, lysis of the microbial body, and cell death.
The best sources of antimicrobial peptides are found in the skin of amphibians.
Over the years, some antimicrobial peptides have been found in the skin of
different amphibian species, including the dermatoxin family, dermaseptin,
plasticin, phyloseptin and cruzioseptin.
In this study, computational methods are used to characterise six cruzioseptin
and four dermsaseptine peptides in order to determine their action mode.
Material e métodos
For this study, various computational techniques were used, highlighting the use
of web servers and software. Pepcal and Biosyn online servers were used for the
prediction of the physicochemical properties such as isoelectric point,
hydrophobicity, hydrophilicity, the number of positively and negatively charged
amino acids, percentage of neutral, acidic, basic, hydrophobic amino acids and
net charge at pH 7 of the respective peptides. The prediction of their secondary
structure was made using Jpred 4, Biosyn and Predictprotein. Subsequently, the
three dimensional secondary structure of the peptides was obtained with Pymol
software, which was then optimized by Gaussian09 software using the ONIOM hybrid
method with the HF/6-31-G basis set. The method and basis set were chosen based
on the number of atoms in the peptides (between 300 and 500 atoms), since the
computational expense required to optimize the peptides with a better method and
with the use of polarized and diffuse bases is very high. Molecular docking was
performed with the help of Autodock Tools software to generate the input files
and perform the calculations in Autodock Vina, thereby obtaining the affinity
values between the peptides and the enzymes and molecules of interest. For the
molecular docking study, the entire molecule was used and not just a region of
the peptides under study.
Resultado e discussão
The physicochemical properties predicted for six cruziospetine and four
dermaseptine peptides show that these peptides has a short chain (21 to 28 amino
acids) with a majority percentage of basic amino acids. The basic amino acids
confer positive charges in excess defining cruziospetines and dermaseptines as
cationic peptides. Also, they have more than 50 % amino acids with hydrophobic
characteristics.
Secondary structure prediction of the mature peptides agrees for all the
software used where cruzioseptins ans dermasptins were predicted to form an
alpha helical structure with an initial and final random coil section.
The mechanism of action of these peptides was studied. Two options were taken
into account. The first one is through inhibition of one or more enzymes of a
vital biological pathway of the bacteria. Second one is cell membrane
lysis caused by charged interaction of the peptides. Two known enzymes of each
studied organism were chosen (E. coli, S. aureus, and C. albicans). Those
enzymes are known as biological targets for several antimicrobial molecules.
Docking results show that, in every case, these peptides have less score (called
affinity in Autodock VINA) compared with their known inhibitor. Therefore, we
can infer that the mechanism of action of the studied peptides is not given by
inhibition of any of the proposed enzymatic pathways as they appear as inactive
molecules towards the enzymes.
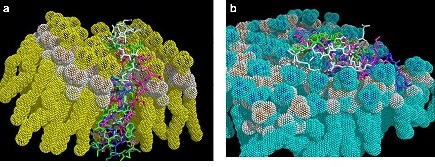
Interaction of four Dermaseptins with a) mammalian cell membrane, b) bacterial cell membrane
Conclusões
Dermaseptins and Cruzioseptins are shortchain cationic peptides that have
different effects on microbes. Physicochemical property prediction showed they are
alpha-helical peptides with isoelectric points greater than 9.7 and a charge that
ranges from + 2 to + 4 at physiological pH. Docking studies suggested that the
possible mechanism of action of this peptide is not given by the inhibition of
vital enzymatic pathways for the microorganism. Instead, by cellular lysis caused
by the interaction of these basic peptides with the negative charged bacterial
cell membrane.
Agradecimentos
Dirección General Académica of the Pontificia Universidad Católica del Ecuador.
Referências
[1] Téllez, G.A.; Castaño, J.C. Péptidos antimicrobianos. Infectio, 2010, vol. 14, no 1, p. 55-67.
[2] Cuesta, S. et al. Modelamiento molecular de la dermaseptina SP2 extraída de
Agalychnis spurrelli. Infoanalítica, [S.l.], v. 7, n. 1, p. 41- 56, ene. 2019. DOI: https://doi.org/10.26807/ia.v7i1.95
[3]Bio-synthesis. 2018. Peptide Property Calculator, Bio-Synthesis Inc. Https://www.biosyn.com/peptidepropertycalculatorlanding.aspx
[4] Drozdetskiy, A., Cole, C., Procter, J. Y Barton G.J. 2015. Jpred4: a protein secondary structure prediction server, Nucleic Acids Research, 43, 389-394.
[5] Rost, B.; Yachdav, G.; Liu, J. The predictprotein server. Nucleic acids Research, 2004, vol. 32, no suppl_2, p. W321-W326.
[6] Frisch, M.J. et al., 2013, Gaussian 09, Revision E.01.
















