Autores
Beltrán-gonzález, S. (UNIVERSIDAD DE CALDAS) ; Rodas-rodríguez, J.M. (UNIVERSIDAD DE CALDAS) ; Rios-vásquez, L.A. (UNIVERSIDAD DE CALDAS) ; Ocampo-cardona, R. (UNIVERSIDAD DE CALDAS)
Resumo
Supported on QTAIM theory and Hirshfeld surface analysis, noncovalent bonding
interactions were studied for three crystalline quaternary ammonium salts QAS
of general structure [(p-YC6H4)2C=CH(CH2)nN(CH2X)(CH3)2]+ I- and their
protein-ligand complex with choline kinase enzyme (ChoK uniprot code P35790).
Then, a study of docking and molecular dynamics was performed and, in turn, a
structural analysis of protein-ligand complexes was carried out using QTAIM
theory. It was found mainly hydrogen type interactions such as H-H and H-π and
H-I in the crystalline systems or H-O in the ChoK-QAS complex. Also, to a lesser
extent, interactions such as π-π and others were found. Calculations were
performed at the HF theory level using the basis set 6-311++G(d,p) or def2-TZVP
for iodine atom.
Palavras chaves
Supramolecular assembly; Complex ChoK-QAS; Dynamics/Docking
Introdução
Although very diverse in its nature, Quaternary Ammonium Salts QAS are widely
used for industrial and medical purposes (EGOROVA et al., P. 7132, 2017), and
some of them are used as blanks in drug design (HERNANDES et al., p. 303, 2017).
QASs type [Ar2C=CH(CH2)nN(CH3)2CH2X]+ I-, have been studied as anti-leishmanial
(DUQUE- BENÍTEZ et al., p. 1, 2016) or anti-Chagas (LÓPEZ-MUÑOZ et al. p. 300,
2019) or antitumor agents (research in progress). From the structural
resemblance of the quaternary ammonium head with choline, a question aims to
understand what region of the molecule plays a more protagonist role on its
bioactivity. As choline kinase enzyme (ChoK) catalyzes the magnesium-mediated
phosphate transfer from ATP to phosphocholine (ÁLVAREZ AYERZA, 2014), higher
phosphocholine levels in comparison to healthy tissue might be often associated
to cancer, so ChoK enzyme becomes an important anti-cancer target (SCHIAFFINO
ORTEGA, 2012).
Computational studies on non-covalent interactions operating in systems similar
to our subjects, it deserves mention the work on ammonium salts coordinated to
molybdenum/zinc (DUTTA et al., 2018; JOHNSTON & AGHO, 2019), or the study of
ammonium salts bearing halogen atoms (DEY et al., 2012, p. 201; JOHNSTON & AGHO,
2019; MEIJIDE et al., 2019; ROSELLÓ et al., 2019). Their approach involves the
study of molecular dynamics and docking, and to a lesser extension,
application of Quantum Theory of Atom in Molecules (QTAIM), or analysis of
Hirshfeld surfaces (DEY et al., 2012, p.201; JOHNSTON & AGHO, 2019; MEIJIDE et
al., 2019; ROSELLÓ et al., 2019).
This work deals with computational calculation of interactions in QAS of
structure [(p-YC6H4)2C=CH(CH2)nN(CH2X)(CH3)2]+I-, both crystalline and as ChoK
protein-ligand complexes.
Material e métodos
Three QASs 1 of general structure [(p-YC6H4)2C=CH(CH2)nN(CH2X)(CH3)2]+ I- were
chosen for the theoretical study: 1a with Y=H, n=2, X= I; 1b with Y=H, n=3, X=
I; 1c with Y=H, n=3, X= I. Their interactions were studied both as crystalline
systems and as ChoK-QAS enzyme-ligand complexes.
Interactions operating in crystalline QASs 1a, 1b and 1c were determined by
analysis of Hirshfeld surfaces using CrystalExplorer software (SPACKMAN et al.,
2021), and by QTAIM as well. Wave functions were obtained by ORCA package
(NEESE, 2012) at HF level with a basis set 6-311++G(d,p) and def2-TZVP for
iodine atom. Then, they were processed with Multiwfn package (LU & CHEN, 2012).
For the study of the respective enzyme-ligand complexes (ChoK-QSA), first step
consisted of 3D modeling of the protein structure using CABS-fold (BLASZCZYK et
al., 2013) and MODELLER software (ŠALI & BLUNDELL, 1993). Then, the study on
enzyme-ligand coupling was performed using each QASs (1a, 1b or 1c) as
substrates with programs Autodock 4 (MORRIS et al., 1998, 2009) and Autodock
Vina (EBERHARDT et al., 2021; TROTT & OLSON, 2010).
Once the complexes were completed, molecular dynamics was carried out during 300
ns with program GROMACS (JAMES ABRAHAM et al., 2015) and the force field
AMBER14SB (MAIER et al., 2015). Parameters for QASs 1a, 1b and 1c were obtained
by ANTECHAMBER (J. WANG et al., 2006) and the force field GAFF (J. WANG et al.,
2004). Trajectories were processed by clustering, and then, wave functions were
calculated from the more representative conformations using ORCA and Multiwfn
for the respective QTAIM analysis. Results were matched using Ligplot package
(WALLACE et al., 1995).
Resultado e discussão
Interactions in crystalline QSAs 1. According with analysis of Hirshfeld
surfaces, the most significant interactions inside crystals involve hydrogen
atoms, with non-covalent contacts associated to H, I, F and C atoms. The nature
of such interactions are mainly I…H, F…H and H…π. On the other hand, QTAIM
analysis allowed not only to verify and quantify the amount of interactions, but
also to identify additional interactions such as I…F, F…π, I…π and π…π, among
others.
Interactions in protein-complex ChoK-QSAs 1. As mentioned above, after
processing the structures of the ChoK-QSA complexes, the analysis of
interactions was performed using LigPlot (as shown in figure 1), suggesting no
hydrogen bond interaction within the active site. This behavior is consistent
for almost all of the clusters formed by the different complexes of the family.
Figure 1
These results were matched with a QTAIM analysis, leading to the conclusion that
the interactions vary widely depending on the kind of salt. Also, it was found
that such interactions may involve a pair of acidic residues (Asp and Glu), a
basic one (Arg) or a great variety of neutral residues (as shown in figure 2).
Figure 2
Furthermore, this analysis strongly suggests that mentioned amino acid residues
involves interactions such as H…H, H…O and H…π, usually found in greater
quantity, with interactions such as I…C, π…π, H…N or I…π being less frequent.
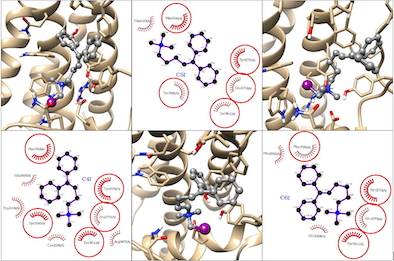
Ligplot evaluation of three representative conformations of QSAs 1
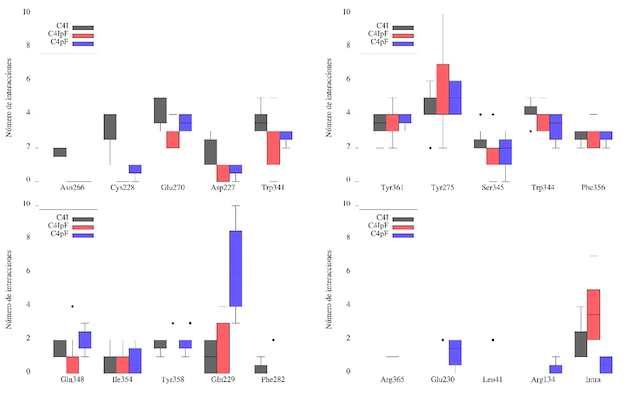
Number of interactions found by QTAIM in QSAs 1
Conclusões
Crystalline interactions involve mainly H type interactions with C, H or iodide,
and their frequency of appearance decrease a little when replaced by I…I-, F…I-
or Cl…I- (if Cl or F are present).
Also, in protein-complexes ChoK-QAS, principal representative interactions
involve H type. Trp, Tyr, Phe and Glu are the residues mainly involved in non-
covalent interactions, mostly H type, i.e H…O, H…pi or H…H.
As expected, I…I, F…I or Cl…I characteristic of crystalline QASs are not observed
anymore in protein-complexes because of iodide anion dilution and molecular
disposition in space.
Agradecimentos
Authors thankfully acknowledge to Universidad de Caldas (Vicerrectoría de
Investigaciones y Postgrados) for the financial support (project code 0430817).
Referências
ÁLVAREZ AYERZA, N. (2014). Mecanismo de acción de los inhibidores de colina quinasa en cáncer de mama. Madrid: Universidad Autónoma de Madrid.
BLASZCZYK, M., JAMROZ, M., KMIECIK, S., & KOLINSKI, A. CABS-fold: Server for the de novo and consensus-based prediction of protein structure. Nucleic Acids Research, 41(W1), W406-W411, 2013
DEY, S. K., DATTA, B. K., & DAS, G. Binding discrepancy of fluoride in quaternary ammonium and alkali salts by a tris(amide) receptor in solid and solution states. CRYSTENGCOMM, 14(16), 5305-5314, 2012
DUQUE-BENÍTEZ, S. M. et al. Synthesis of Novel Quaternary Ammonium Salts and Their in Vitro Antileishmanial Activity and U-937 Cell Cytotoxicity. Molecules 21, 1–16, 2016
DUTTA, D., NASHRE-UL-ISLAM, S. M., SAHA, U., CHETRY, S., GUHA, A. K., & BHATTACHARYYA, M. K. Structural Topology of Weak Non-covalent Interactions in a Layered Supramolecular Coordination Solid of Zinc Involving 3-Aminopyridine and Benzoate: Experimental and Theoretical Studies. Journal of Chemical Crystallography, 48(4), 156-163, 2018
EGOROVA, K. S., GORDEEV, E. G., ANANIKOV, V. P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 117, 7132–7189, 2017
EBERHARDT, J., SANTOS-MARTINS, D., TILLACK, A. F., & FORLI, S. AutoDock Vina 1.2. 0: New docking methods, expanded force field, and python bindings. Journal of Chemical Information and Modeling, 61(8), 3891-3898, 2021
FERNANDES, M. Z., CAVALCANTI, S. M. T., MOREIRA, D. R. M., DE AZEVEDO, J., FILGUEIRA, W., & LEITE, A. C. L. Halogen atoms in the modern medicinal chemistry: Hints for the drug design. Current drug targets, 11(3), 303-314, 2010
JAMES ABRAHAM, M., MURTOLA, T., SCHULZ, R., PÁLL, S., SMITH, J. C., HESS, B., & LINDAHL, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX, 1-2, 19-25, 2015
JOHNSTON, D. H., & AGHO, I. Crystal Structures and Hydrogen-Bonding Analysis Of A series of solvated ammonium salts of molybdenum (II) chloride clusters. Acta Crystallographica Section E: Crystallographic Communications, 75(11), 1705-1711, 2019
LÓPEZ-MUÑOZ, M., GÓMEZ-PEÑA, J. J., RÍOS-VÁSQUEZ, L. A. et al. Novel fluorinated quaternary ammonium salts and their in vitro activity as trypanocidal agents. Med. Chem. Res. 28, 300–319, 2019
LU, T., & CHEN, F. Multiwfn: A multifunctional wavefunction analyzer. Journal of Computational Chemistry, 33(5), 580-592, 2012
MAIER, J. A., MARTINEZ, C., KASAVAJHALA, K., WICKSTROM, L., HAUSER, K. E., & SIMMERLING, C. Ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from ff99SB. Journal of Chemical Theory and Computation, 11(8), 3696-3713, 2015
MEIJIDE, F., VÁZQUEZ-TATO, M. P., SEIJAS, J. A., DE FRUTOS, S., TRILLO NOVO, J. V., SOTO, V. H., & VÁZQUEZ TATO, J. Crystal Structure of a Cationic Bile Salt Derivative ([3β,5β,7α,12α]-3-(2-naphthyloylamino)-7,12-dihydroxycholan-24-triethylammonium iodide). Crystals, 9(3), 135, 2019
MORRIS, G. M., GOODSELL, D. S., HALLIDAY, R. S., HUEY, R., HART, W. E., BELEW, R. K., & OLSON, A. J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. Journal of computational chemistry, 19(14), 1639-1662, 1998
NEESE, F. Orca 4.2. 1. Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2, 73, 2012
ROSELLÓ, Y., BENITO, M., MOLINS, E., BARCELÓ OLIVER, M., & FRONTERA, A. Adenine as a Halogen Bond Acceptor: A Combined Experimental and DFT Study. Crystals, 9(4), 224, 2019
ŠALI, A., & BLUNDELL, T. L. Comparative Protein Modelling by Satisfaction of Spatial Restraints. Journal of Molecular Biology, 234(3), 779-815, 1993
SCHIAFFINO ORTEGA, S. (2012). Nuevos inhibidores de colina quinasa no simétricos y simétricos más polares con actividad antitumural y antimalárica. Universidad de Granada.
SPACKMAN, P. R., TURNER, M. J., MCKINNON, J. J., WOLFF, S. K., GRIMWOOD, D. J., JAYATILAKA, D., & SPACKMAN, M. A. ıt CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. Journal of Applied Crystallography, 54(3), 1006, 2021
TROTT, O., & OLSON, A. J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of computational chemistry, 31(2), 455-461, 2010
WALLACE, A. C., LASKOWSKI, R. A., & THORNTON, J. M. LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein engineering, design and selection, 8(2), 127-134, 1995
WANG, W., BAI, Z., ZHANG, F., WANG, C., YUAN, Y., & SHAO, J. Synthesis and biological activity evaluation of emodin quaternary ammonium salt derivatives as potential anticancer agents. European journal of medicinal chemistry, 56, 320-331, 2012
WANG, J., WANG, W., KOLLMAN, P. A., & CASE, D. A. Automatic atom type and bond type perception in molecular mechanical calculations. Journal of molecular graphics and modelling, 25(2), 247-260, 2006
WANG, J., WOLF, R. M., CALDWELL, J. W., KOLLMAN, P. A., & CASE, D. A. Development and testing of a general amber force field. Journal of Computational Chemistry, 25(9), 1157-1174, 2004
















